| Posted: December 3, 2008 |
Mass production technique for metal-complex-type organic nanotubes |
|
(Nanowerk News) Scientists at the National Institute of Advanced Industrial Science and Technology (AIST) in Japan have developed a new technique for the mass production of unique metal-complex-type organic nanotubes. It was found that the nanotubes can be obtained when an aqueous metal salt solution is added to an alcoholic suspension of amphiphilic molecules comprising glycylglycine and fatty acids. In this process, the sheet-like structure of the amphiphilic molecules was found to change to a nanotube structure within 10 minutes. This method of synthesis has been extended to the mass production of organic nanotubes. The new technique is almost five times faster than the conventional method, and the amount obtained is 200 times that obtained in the conventional method.
|
|
AIST has developed two kinds of organic nanotubes with hydroxyl groups or carboxyl groups on their surfaces, which can be mass produced. Metal-complex-type organic nanotubes, in which metal ions are present on the surface, are the third example of organic nanotubes produced in large quantities.
|
|
This new class of organic nanotubes with metal ions on their inner and outer surfaces and within their membranes are expected to function as efficient catalysts because of their large specific surface areas; they can also be used to separate functional materials such as DNA and proteins. Metallic nanostructures, where nanotubes are used as templates, may find applications as electronic, magnetic, and optical materials. The organic nanotubes with metal ions are expected to be used in various fields such as medical and health care, food industry, biotechnology and electronics.
|
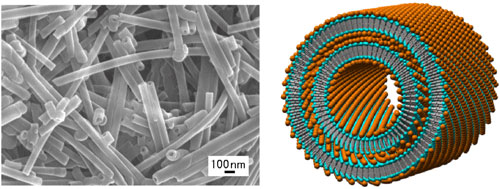 |
| (Left) FE-SEM image of the metal-complex-type organic nanotubes and (Right) their proposed structure. Metal ion is designed as an orange ball, a hydrophilic part of the amphiphile as a light blue ball, and a hydrophobic part of the amphiphile as a gray stick. (Image: AIST)
|
| The results of this research will be exhibited at Nanotech 2009, which will be held at Tokyo Big Sight on February 18–20, 2009.
|
|
Background for Research
|
|
Under the present circumstances in which the steep rise in the prices of oil and resources pose serious social problems, there is an immediate demand for the development of a manufacturing technique that consumes less energy, which uses the advantage of self-assembly, for example, and nanotechnology that does not deplete energy resources. Hence, raw materials obtained from renewable natural are preferred over those obtained from petroleum sources.
|
|
History of Research
|
|
For more than a decade, AIST has been conducting researches on the synthesis of organic nanomaterials, including nanofibers and nanotubes, through the self-assembly of amphiphilic molecules synthesized from renewable natural product sources such as sugars, peptides, nucleic acids, fats and oils in solution, and has kept a high potential in these technological fields. In 2006, for the first time in the world, AIST succeeded in the mass production of organic nanotubes, and is now focusing on the application of these nanotubes in various fields.
|
|
Glucose-type and glycylglycine-type organic nanotubes can be produced in large quantities, and they have hydroxy groups and carboxyl groups on their surfaces, respectively. Therefore, these nanotubes can be used as encapsulating, adsorbing, or separating agents only for target molecules that have large interaction with hydroxy or carboxyl groups.
|
|
On the other hand, researchers at AIST have found that glycylglycine-type amphiphilic molecules (hereafter referred to as "peptide lipids") form organic nanotubes in water even though they form metal complexes and that the metal ions exist on the surface of the nanotubes thus formed. However, because of the low solubility of the peptide lipids in water, only approximately 0.5 g of the metal-complex-type organic nanotubes could be formed from 1 liter of water.
|
|
This research was carried out as part of the Solution Oriented Research for Science and Technology (SORST) project, the contract research of Japan Science and Technology Agency (FY 2005 - 2008).
|
|
Details of Research
|
|
In this research, we found that we could obtain organic nanotubes consisting of metal complexes by adding an aqueous solution of metal salts to a suspension of peptide lipids in alcohol. The sheet-like structure of the peptide lipids suspended in alcohol changed to the nanotube structure within 10 minutes of addition of the metal salt solution.
|
|
In the conventional method of nanotube formation, the peptide lipids are dissolved in water by addition of 1 equivalent of alkali and subsequent ultrasonication and warming at approximately 40 °C. Despite these efforts, only 0.01 g of the peptide lipids could be dissolved in 20 mL of water. On the other hand, in our new method, peptide lipids can be easily suspended in alcohol by simple stirring. Thus, this method consumes less energy and is highly convenient. Since solubility of the peptide lipids is not a limiting factor, the yields are dramatically increased. As of now, it has been possible to form 2 g of nanotubes from 20 ml of alcohol, which implies that the yield is almost 200 times that obtained in the conventional method. Further enhancement of the yield also appears possible.
|
|
The metal-complex-type organic nanotubes that have been synthesized in bulk have metal ions on their surfaces. They are the third example of such organic nanotubes, the first two being organic nanotubes with hydroxy groups and carboxyl groups on their surfaces. All metal ions that can coordinate with carboxylate anions (-COO-) can be used for synthesizing these organic nanotubes. Metal ions that have been successfully used for nanotube synthesis at present include Zn2+, Cu2+, Co2+, Ni2+, Fe2+, and Mg2+.
|
|
Since our organic nanotubes have metal ions on their inner and outer surfaces, they can be used as encapsulating, adsorbing, or separating agents for functional materials, including drugs, which are not compatible with organic nanotubes having hydroxy or carboxyl groups on their surfaces. An example of one of these applications is the selective adsorption of gold nanoparticles having surface amino groups (-NH2) on copper-complex-type organic nanotubes. These organic nanotubes are also expected to be useful for the adsorption or separation of biopolymers such as DNA and proteins.
|
|
Metallic nanostructures obtained using metal-complex-type organic nanotubes as templates may find applications as electronic, magnetic, and optical materials. We have succeeded in fabricating metal-oxide nanotubes by sintering metal-complex-type organic nanotubes in air and organic nanotubes with silver nanoparticles embedded in their membranes by photoreduction of silver-complex-type organic nanotubes.
|
|
Future Research
|
|
Metal-complex-type organic nanotubes are nanomaterials with a novel structure, and though their concrete applications have not been identified as yet, they have potential applications in various fields such as medical and health care and in nanobiotechnology as encapsulating, adsorbing, and separating agents for low-molecular-weight molecules having functional groups that can coordinate with metals, DNA, and proteins. They can also be used as catalysts using transition metal coordinating to the nanospace. They can be converted to metal-oxide nanotubes or hybrid nanotubes in which metal nanoparticles are dispersed and then used as electronic, magnetic, and optical materials.
|

