| Posted: February 19, 2009 |
A bright new approach to directing nanoparticle self-assembly |
|
(Nanowerk News) For researchers on the cutting edge of chemistry and materials science, learning how to manipulate particles hundreds of times smaller than the diameter of a human hair and how to direct their self-assembly in a predictable way has been a formidable but exciting challenge.
|
|
One of the brightest new approaches, reported in the current issue of Nature, comes from chemists Vincent Rotello and Bappaditya Samanta of the University of Massachusetts Amherst, with materials scientist Ben Yellen and co-workers at Duke University. They have coaxed three different-sized particles suspended in a colloidal liquid to self-assemble into elegantly shaped structures reminiscent of flowers and Saturn, complete with rings, by applying a controlled static magnetic field.
|
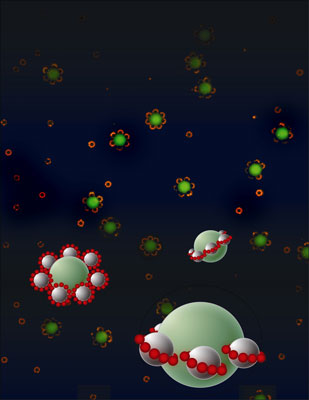 |
| The UMass-Amherst/Duke University researchers guided assembly of many different types of rotationally symmetric nanostructures, including axial quadrupoles or Saturn rings, from simple colloidal building blocks using a new technique involving ferrofluid.
|
|
The work, supported by the National Science Foundation, is described in the Feb. 19 issue of Nature. It could lead to the development of sophisticated new ways to organize groups of cells for medical applications or to produce customized nanostructures for advanced optical devices. But even without a purpose in mind as yet, Rotello notes, the UMass Amherst-Duke team has provided “a new method that people hadn’t thought of before, and it opens many new possibilities. What’s going to be useful will be the new structures we can assemble.”
|
|
As Rotello explains, “With previous methods, you could usually get one thing to assemble, or you may get two, but we’ve been able to get three particles at one time to assemble in a hierarchical manner, and we’re working on more.” By hierarchical, he means layered or organized rather than randomly distributed.
|
|
The tiny nonmagnetic particles in these experiments, each with its own unique magnetic identity, float aimlessly in the specially designed ferrofluid that contains iron oxide. But once directed by a controlled external magnetic field, “everything assembles,” Rotello explains. “When you turn the field off, everything wanders off. The fluid acts like a super-small sheepdog. In response to the master’s whistle it not only herds the particles into coherent clusters but at the same time, segregates three different species into patterned groups.”
|
|
The resulting nanostructures self-assemble to form rotationally symmetric superstructures the chemist calls “simply beautiful.” Under the microscope some look like flower petals, others resemble Saturn and its rings. By tuning the strength and other properties of the magnetic field and varying ferrofluid characteristics, the researchers are learning to control interactions in the colloidal suspension, guiding nanostructure formation. Further, using heat and certain chemicals as “glue,” the chemists have developed a way to permanently link or fix the structures so they will not come apart and lose their structural usefulness when the magnetic field is turned off.
|
|
Rotello and colleagues say they may next turn to test the new method in biological systems. “We may see if we can get cells to do the same kind of dance we’ve gotten the particles to do,” he notes. They also plan to test different combinations of particles types and sizes, as well as ferrofluid composition, to see how small they can make new nanostructures.
|

