| Aug 21, 2012 |
Nanotechnology scientists create new diamond-denting carbon
|
|
(Nanowerk News) A new super-hard form of carbon has been created by an international team of scientists working with X-rays at the Advanced Photon Source at the Department of Energy’s Argonne National Laboratory (see paper in Science: "Long-Range Ordered Carbon Clusters: A Crystalline Material with Amorphous Building Blocks").
|
|
Carbon materials, such as graphene, graphite, buckyballs and nanontubes, display a remarkable range of mechanical, electronic and electrochemical properties that make them sought-after materials for advanced products in electronics and nanotechnology.
|
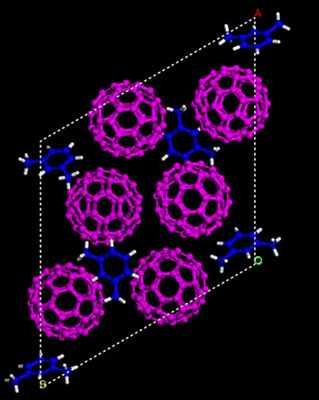 |
| Simulated structures showing the starting material of carbon-60 “buckyballs” (magenta) and m-xylene solvent (blue) before being compressed.
|
|
Scientists with the Carnegie Institute of Washington’s Geophysical Laboratory led the research team, which was also comprised of scientists from Argonne, Jilin University, the University of Nebraska at Lincoln, Stanford University and SLAC National Accelerator Laboratory. Their work was conducted at the High Pressure Collaborative Access Team at the Advanced Photon Source.
|
|
"We created a new type of carbon material, one that is comparable to diamond in its inability to be compressed," said lead author and Carnegie scientist Lin Wang. "Once created under extreme pressures, this material can exist at normal conditions, meaning it could be used for a wide array of practical applications."
|
|
Not only is the new material incredibly strong – it can dent diamond, the hardest substance on Earth – but also it was able to retain its new super-hard form even when the high pressure that created it was removed.
|
|
The new material combines two forms of carbon, one with an organized atomic structure and one without, into a hybrid material only previously theorized about. Wang’s team crushed hollow carbon-60 molecules called buckyballs between two flattened diamond tips until the molecules collapsed into a new harder, carbon form. They found a sweet spot in the pressure level where the new material held its form and did not return to its less durable buckyball state. That optimal crushing pressure is 320,000 times atmospheric pressure. The ability to maintain super-hard status without continual pressure is key for commercial applications.
|
|
X-ray diffraction, Raman spectroscopy, infrared absorption spectroscopy and inelastic X-ray scattering were used to analyze the materials crystal structure, lattice vibration and bonding type at high pressure.
|
|
“The results are exciting,” said Yang Ding, a coauthor of the paper and a scientist with the Advanced Photon Source. “The new form of carbon may have some new properties which others do not have due to its novel structure.”
|
|
"The thing that stands out for me from this work," Lin Wang added, "is that carbon-60 can crystallize with various solvents, and those solvates would have different periodicities, which enables us to synthesize a series of similar carbon materials with different packing symmetry and carbon cluster size by compressing different types of carbon molecules."
|

