| Sep 13, 2013 |
A nanoscale glimpse of batteries in action
|
|
(Nanowerk News) Lithium–oxygen (Li–O2) batteries are a new type of experimental battery that electric car manufacturers are hoping will address the issue of limited driving range. Unlike the lithium-ion batteries used today, lithium–oxygen batteries do not require metal oxide cathodes to produce electrochemical power, instead generating power from reactions with oxygen in the atmosphere. The significant weight savings realized through this design could potentially boost energy densities of batteries by up to four times. However, lithium–oxygen batteries have yet to leave the laboratory due to short battery lifespans caused by parasitic side reactions and accumulated charge polarization at battery cathodes.
|
|
Hye Ryung Byon with colleagues Rui Wen and Misun Hong from the RIKEN Byon Initiative Research Unit have now captured never-before-seen details of lithium–oxygen reactions using in situ atomic force microscopy (AFM) as a step toward resolving these drawbacks ("In Situ AFM Imaging of Li–O2 Electrochemical Reaction on Highly Oriented Pyrolytic Graphite with Ether-Based Electrolyte").
|
 |
| Figure 1: High-resolution microscopy reveals that lithium-oxygen batteries discharge power through a series of nanoscale product growth processes on atomically flat HOPG surfaces. (©2013 American Chemical Society)
|
|
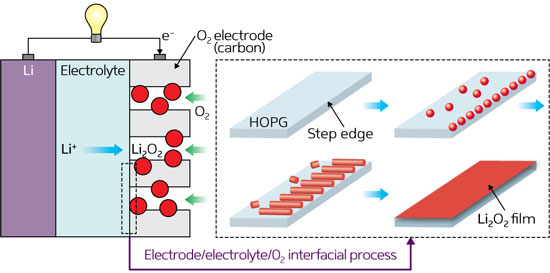
Byon and her colleagues set out to gather unambiguous evidence of nucleation, growth and decomposition of lithium–oxygen electrochemical products using AFM to trace out surface topographies down to nanometer scales. In their study, they crafted a model cell comprised of a highly oriented pyrolytic graphite (HOPG) working electrode, metallic lithium counter and reference electrodes, and an ether-based solvent packed with oxygen gas and lithium salts as an electrolyte.
|
|
As lithium–oxygen batteries discharge power, lithium ions react with oxygen to produce solid lithium peroxide (Li2O2) deposits on atomically flat HOPG substrates. When the team monitored this reaction by AFM, they saw tiny particulates less than 10 nanometers high begin to nucleate along the ‘step edges’ inherent to HOPG (Fig. 1). These nanoparticles swiftly grew into elongated ‘nanoplate’ structures of micrometers in length, and further reaction caused the nanoplates themselves to fuse into a new lithium peroxide film.
|
|
The researchers then observed the battery recharge process, where oxygen gas is released and lithium ions migrate to the metallic lithium electrodes. Their AFM images revealed that during the initial recharge cycle, the lithium peroxide film decomposes completely, leaving no residue on the HOPG. By the fifth cycle, however, many by-products emerged as a result of the oxidation of the HOPG electrode and degradation of the ether-based electrolyte and lithium salt.
|
|
Byon notes that these findings will help researchers accurately correlate parameters such as electrode composition and size with changes in battery performance. “This could provide insight into the design of catalysts and electrodes for lithium–oxygen batteries,” she says.
|

