| Jul 25, 2023 | |
Biomolecular nanomotors for precision medicine |
|
| (Nanowerk Spotlight) Nanomedicine, a field that has seen enormous strides since its inception in 1999, is focused on improving therapeutic agents through the exploitation of nanoscale properties of materials. Over the past two to three decades, a myriad of nanoparticle systems of varying shapes, sizes, and compositions have been tested and explored in vitro, ex vivo, and in vivo. | |
| However, despite extensive investments of time, money, and resources, only 32 nanoparticle treatments have been approved for clinical use so far. One of the reasons for this is the lack of focus on treatment outcomes. What is needed is a deeper understanding of the fundamental biology to provide a framework for rational nanomedicine drug design. | |
| The application of nanomotors in cancer diagnosis and therapy is a burgeoning field of research that, when combined with precision nanomedicine, promises to address many of the challenges faced by the previous generation of passive nanoparticles. | |
| A recent review in Advanced Materials ("A Biomolecular Toolbox for Precision Nanomotors") aims to introduce researchers to the wealth of knowledge available regarding cancer cell biology and biochemistry, as well as to provide a deeper understanding of the complexity of cell membrane compositions, extracellular surfaces, and their functional consequences. | |
| The progression from passive nanoparticles to active nanomotors, and from active nanomotors to precisely targeted active nanomotors, is hoped to bring about a paradigm shift in the nanomedicine field. A successful clinical implementation of active precision nanomotors is expected to represent an enormous leap, not only in materials and biomedical science, but also in the treatment of deleterious diseases that are difficult to treat today. | |
The Promise of Nanomotors |
|
| Nanomotors, defined as nano-scale constructs that utilize energy from their surrounding environment for motility, are one of the newest developments in the ever-evolving landscape of nanoparticle research. The enhanced mobility and promise of directed targeting provided by active nanomotors have expanded the opportunities available for precision nanomedicine. This is particularly the case for chemotherapies whose general toxic side-effects can be ameliorated by targeted delivery to the cancer. | |
| From the moment of application into a body, nanomotors must face many barriers before being able to reach the intended target. These can be broadly classified into two groups: physical barriers such as diffusion and shear forces, and biological barriers, such as phagocytosis, renal clearance, extracellular matrices, and neutrophil extracellular traps. Nanomotors with enhanced mobility may be able to address issues surrounding the barriers to nanoparticle delivery. This is particularly important in cancer, as tumors are known to have higher interstitial pressures and poorer vascularization than healthy tissue, which makes passive delivery of drugs or treatments problematic. | |
| The different nanomotors available to nanomedicine drug development can be categorized by their propulsion systems. Each propulsion method has its own set of advantages, disadvantages, and incompatibilities, which can influence their suitability for different applications. | |
| Chemical Propulsion | |
| Chemical propulsion is a method used to drive nanomotors, and it involves the use of catalysts or enzymes to degrade a local fuel source, such as a gas or chemical gradient, in the microenvironment. The by-products of this reaction provide motion. This method doesn't require external equipment and has intrinsic propulsion capabilities. However, it does require a fuel source and can create bubbles and potentially toxic by-products of reaction. | |
| Many diverse morphologies and strategies have been applied for chemically propelled nanomotors. For example, Janus nanomotors, which are named for their two-faced structure, have been used. These nanomotors have specific geometries designed to retain or expel products in order to provide directional motion. | |
| One example of a chemically propelled nanomotor involves the use of catalysts such as noble metals (gold, platinum), and enzymes such as catalase and glucose oxidase. For instance, a nanomotor was created using poly(2-(dimethylamino)ethyl methacrylate) (PDPA) and catalase in zinc imidazole framework-L (ZIF-L) metal organic frameworks (MOFs). At a mildly acidic pH, as can be found in the tumor microenvironment, the nanomotor undergoes a reversible gelation process which prevents access of fuel to catalase, drastically reducing micromotor motion. | |
| Another example involves the use of glucose oxidase. Glucose is a safe and much more common nutrient found in the body, which can be decomposed into H2O2 and d-glucono-1,5-lactone by glucose oxidase. Therefore, glucose oxidase can be used in tandem with catalase or another catalyst. This tandem enzyme method has been explored by encapsulating photosensitive nanoparticles with ZIF-8 metal organic frameworks (MOFs), and functionalizing them with glucose oxidase and catalase. | |
| Acoustic/Ultrasound Propulsion | |
| Acoustic or ultrasound propulsion is a non-invasive energy delivery method that offers the advantage of being able to focus on a small area due to the wavelengths used. Ultrasonically powered nanomotors commonly use gold nanowires, which can be functionalized in various ways to enhance cell uptake. | |
| One example of an ultrasonically propelled nanomotor is a gold nanowire loaded with silencing RNAs (siRNA) for GFP silencing. This demonstrates the potential for using ultrasonically propelled nanomotors for gene silencing applications. | |
| Another example of an acoustic propulsion system involves the use of conical tubular nanomotors coated with a gold nanoshell at the conical opening. These nanomotors have been directed for drug delivery using an adjustable ultrasonic field. These nanomotors have been shown to be capable of cell membrane perforation by irradiation with near-infrared light. | |
| However, there are some limitations to consider with acoustic or ultrasound propulsion. For instance, due to the complexity of the geometry of tissues and vasculature, acoustic standing waves cannot be used for nanomotor propulsion, although traveling standing waves can be used. Also, the effectiveness of propulsion in tissue involves a compromise between penetration depth and spatial resolution. Higher frequency ultrasound produces higher resolution directional control of nanomotors, but cannot penetrate thick tissue, whereas lower frequency ultrasound can penetrate deeper but with less resolution. | |
| Light-based Propulsion | |
| Light-based propulsion, also known as photonic propulsion, is another method used to drive nanomotors. This method involves the use of light to generate motion in photoactive or photothermal materials. There are several mechanisms through which light can induce motion in these materials, including the generation of heat (thermophoresis), ions (self-electrophoresis/electrolyte diffusiophoresis), bubble propulsion, and mechanical deformation. | |
| However, the high ionic strength of biological fluids precludes the use of self-electrophoretic or diffusiophoretic mechanisms. Bubble generation is also not desirable except in certain applications. Therefore, the most commonly used mechanism in light-based propulsion is thermophoresis, which involves the generation of heat. | |
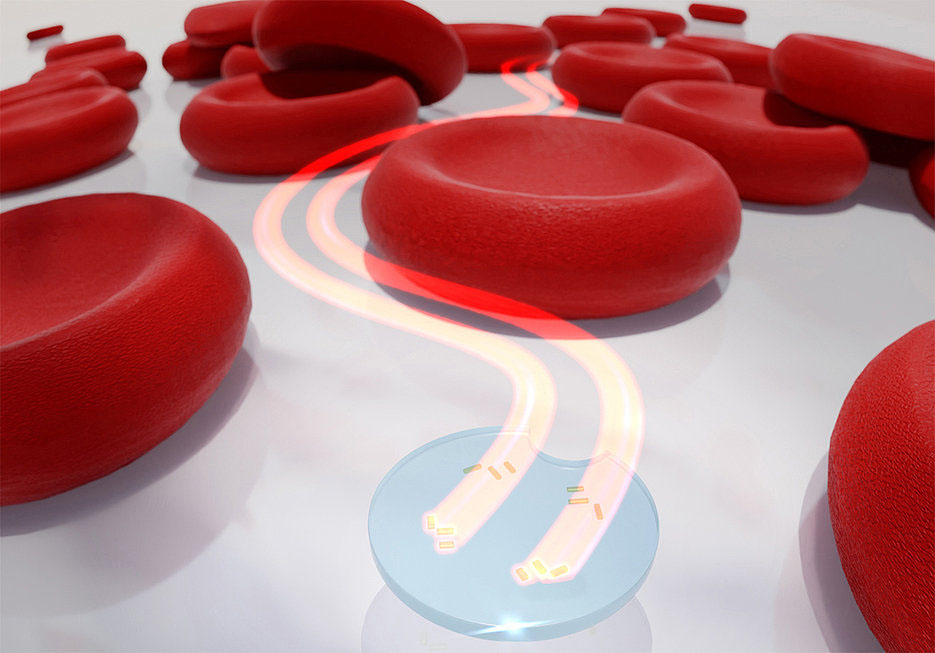 |
|
| Artistic representation of a microdrone with two active light-driven nanomotors between red blood cells. (Image: Thorsten Feichtner, Universität Würzburg) | |
| One example of light-based propulsion is the use of self-thermophoretic Janus nanomotors. These are constructed out of azobenzene and poly(D,L-lactide) (PLA), creating a cargo-carrying polymer engine that undergoes reversible elongation, non-diffusion movement, and heating under UV irradiation. After loading the nanomotors with Nile Red, cell uptake experiments showed enhanced uptake in a clathrin-dependent manner. | |
| Another example is the use of multilayer nanomotors that convert near-infrared light into blue light for photodynamic therapy. These nanomotors are also functionalized with glucose oxidase, which serves to simultaneously starve tumor cells of glucose as well as provide fuel for autonomous locomotion. When HeLa cells were treated with these nanomotors and irradiated with a 980 nm laser, there was a significant reduction in cell viability. | |
| Magnetic Propulsion | |
| Magnetic nanomotors are guided by magnetic fields. They offer excellent depth of penetration, are non-invasive, and can be precisely controlled. However, they also require bulky equipment. Patients with metal implants may also be incompatible with this type of propulsion, depending on the type of implant and the strength of the magnetic field. | |
| Biohybrid Nanomotors | |
| Biohybrid propulsion is a unique method of nanomotor propulsion that combines biological components with synthetic materials. This approach leverages the natural locomotion abilities of certain entities like bacteria, algae, immune cells, and sperm cells. | |
| Bacteria-based biohybrid micromotors have been extensively studied. In one instance, bacteria were bound to water-oil-water double microemulsions loaded with fluorescent dyes via streptavidin-biotin conjugation. These bacteria could then migrate down a glucose gradient, demonstrating the potential for targeted delivery. | |
| Another example of a biohybrid micromotor involves the use of bovine sperm. The sperm were embedded in a "tetrapod" construct consisting of four flexible arched arms surrounding a tubular body. This structure was fabricated using two-photon 3D nanolithography and coated with a 10 nm iron layer. The head of the sperm cells becomes lodged inside the body of the tetrapod, while the tail is free to rotate and provide propulsion. By applying an external magnetic field, the hybrid nanomotor can be steered towards a target. The sperm cells were loaded with doxorubicin by co-incubation, which had no significant effect on sperm viability. | |
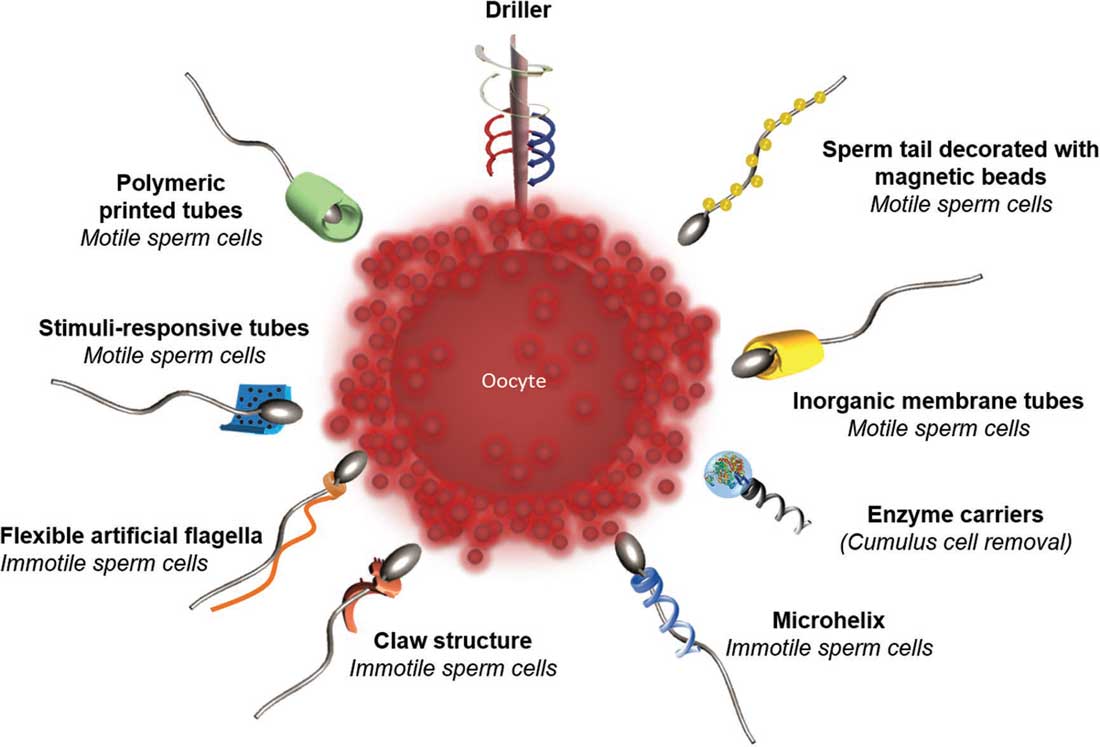 |
|
| Spermbots – ovel microswimmer designs for targeting fertilization. From the top and clockwise: magnetically actuated drillers for penetrating the cumulus layer of the oocyte; the sperm tail decorated with magnetic beads for magnetic actuation of the tail. Rolled up microtubes made of inorganic nanomembrane for the guidance of motile sperm cells; magnetic helical enzyme carriers for the cumulus cell removal by enzymes (e.g., hyaluronidase); artificial flagella with multiple windings and a claw-shaped sperm carrier for the delivery of immotile sperm cells; artificial flexible flagella for transport of immotile spermatozoa; stimuli-responsive polymeric microtubes for the delivery of motile spermatozoa; and polymeric printed microtubes for the guidance of motile sperm cells. (Reprinted with permission by Wiley-VCH Verlag) | |
| Red blood cells have also been used in biohybrid nanomotors. They were loaded with magnetic nanoparticles, quantum dots, and doxorubicin for controllable drug delivery and imaging. Despite the potential of biohybrid propulsion, the complexity of these multivariate targeting schemes increases the level of difficulty for clinical application. Furthermore, the safety of these biohybrid micromotors is still preliminary and needs further investigation before they can be deemed useful for clinical applications. | |
| The authors then delve into the various molecular strategies that can be employed to achieve specific targeting and high affinity binding in the context of nanomotors. | |
| Antibodies | |
| Antibodies are proteins produced by the immune system in response to exposure to foreign antigens. They bind specific antigens with high affinity and specificity. Structurally, they consist of two variable (Fab) regions, which enable binding to a target region of the antigen. The use of antibodies for targeting in nanomedicine is well established, and several bioinformatic tools exist for the design and prediction of cell penetrating peptides, which will be useful in the delivery of therapeutic payloads in nanomotor systems. | |
| Nanobodies | |
| Nanobodies are a type of antibody that are significantly smaller than conventional antibodies. They are derived from heavy-chain-only antibodies found in camelids and sharks. Nanobodies have been used for targeting in nanomedicine due to their small size, high stability, solubility, and ease of production in bacteria. Protein engineering also allows for the construction of dimeric nanobody constructs, with either two of the same nanobody, two nanobodies recognizing two epitopes of the same antigen, or even two nanobodies recognizing two different antigens. | |
| Aptamers | |
| Aptamers are single-stranded oligonucleotides that can bind to a target with high affinity and specificity. They are selected from a pool of random sequences through a process called SELEX (Systematic Evolution of Ligands by Exponential Enrichment). The size of this oligonucleotide pool is in the order of 10^14–10^16 strands, with fixed sequences on both ends, as well as a randomized sequence in the middle. Unbound sequences are removed, and ligand-binding sequences are amplified by polymerase chain reaction (PCR). These are then subjected to several rounds of selection and amplification to enrich for sequences that bind to the target with high affinity. | |
| Peptides | |
| Peptides are short chains of amino acids that can be used for targeting in nanomedicine. They can be designed to mimic the binding sites of proteins, and can be easily synthesized and modified. Several bioinformatic tools exist for the design and prediction of cell penetrating peptides, which will be useful in the delivery of therapeutic payloads in nanomotor systems. | |
| Bonding Strategies | |
| The bonding strategies between a nanomotor and a targeting molecule can be broadly classified into covalent, non-covalent, and affinity-based bonds, which are strong, intermediate, and weak respectively. The most stable and reliable bonding between a nanomotor and targeting molecule is covalent bonds. The exceptions to be made are biotin-streptavidin bonding, sulfur-metal bonding, and histidine-metal bonding, which are affinity-based but have high enough affinities to be considered pseudo-covalent. | |
| Concluding their review, the authors acknowledge the enormous diversity in the field of nanomedicine and nanomotors, which they view as an encouraging sign for the development and refinement of new ideas and technologies. They emphasize that we are entering a new age where many types of new exotic materials of various compositions, classes, and chemistries can be guided by fundamental biology, science, and the insights gleaned thereof. | |
| The authors stress the importance of closer collaboration with clinicians in the development of nanomotors to accelerate the translation of nanomotor research into clinically useful cancer treatments. They also highlight the need for a deeper understanding of the fundamental biology to provide a framework for rational nanomedicine drug design. Consideration must be given to the regulatory framework, as well as the needs of clinicians and patients. | |
| Despite the promise of nanomedicine research, the authors note that it remains to be fully realized. They point out that one of the reasons for this is the lack of focus on treatment outcomes. They argue that what is needed is a deeper understanding of the fundamental biology to provide a framework for rational nanomedicine drug design. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
