Nanosensors – what they are; what they do
Nanosensors are tiny sensors that are designed to measure physical, chemical, biological, or environmental information at the nanoscale level and transfer it into data for analysis. They are made from nanomaterials and have unique properties, such as high surface-to-volume ratio, that make them ideal for sensing applications. Nanosensors are used in a wide range of fields, such as healthcare, environmental monitoring, and security, and have the potential to revolutionize the way we detect, measure, and respond to various signals and stimuli.
Nanotechnology deals with physical or chemical properties of matter at the nanoscale, which can be different from their bulk properties. Nanosensors can take advantage of these phenomena. Important characteristics and quality parameters of nanosensors can therefore be improved over the case of classically modeled sensors with merely reduced sensing parts and/or the transducer.
Therefore, nanosensors are not necessarily reduced in size to the nanoscale, but could be larger devices that make use of the unique properties of nanomaterials to detect and measure events at the nanoscale. For instance, in noble metals such as silver or gold, nanostructures of smaller size than the de Broglie wavelength for electrons lead to an intense absorption in the visible/near-UV region that is absent in the spectrum of the bulk material.
Nanosensors have been developed for the detection of gases, chemical and biochemical variables, as well as physical variables and the detection electromagnetic radiation.
Nanosensor fabrication
Nanosensors can be prepared by using different methods. Three common methods are top-down lithography, bottom-up fabrication (such as for instance controlled lateral epitaxial growth and atomic layer deposition), and self-assembled nanostructures (usually done with biomolecules, e.g. liposomes, that combine in such a way that the biochemical detection of an analyte is converted into an electrical signal).
Nanosensors based on nanoparticles and nanoclusters
Nanoparticles, primarily noble metal ones, have outstanding size-dependent optical properties that have been used to build optical nanosensors.
The spectrum of a phenomenon called the localized surface Plasmon resonance (LSPR) depends on the size, shape and material of the nanoparticle itself as well as the particle's environment. The high sensitivity of LSPR sensors can approach the single-molecule limit of detection for large biomolecules.
Apart from metal nanoparticles, optical nanosensors based on fluorescence measurements have been built with semiconductor quantum dots and other optical sensors have been developed with nanoscale probes that contain dyes whose fluorescence is quenched in the presence of the analyte to be determined; nanoparticle films have been used for gas sensors; magnetic nanoparticles bound to biorecognitive molecules (i.e. DNA, enzymes, etc.) have been used to enrich the analyte to be detected.
For example, researchers have developed an enzyme biomarker test based on gold nanoparticles that can detect enzyme markers of disease known as proteases in humans, animals and food products. This nanosensor indicates when proteases are present through a visible color-change reaction.
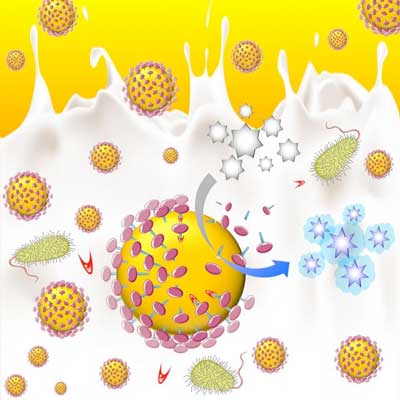
Gold nanoparticles covered in casein. Proteases are present, they 'eat' the protective casein barrier, exposing the surface of the gold nanoparticles. (Image: Queen's University Belfast)
Nanosensors based on nanowires, nanofibers and carbon nanotubes
Most sensors based on carbon nanotubes (CNTs) are field effect transistors (FET) because, although CNTs are robust and inert structures, their electrical properties are extremely sensitive to the effects of charge transfer and chemical doping by various molecules. The functionalization of CNTs is important for making them selective to the target analyte – different types of sensors are based based on molecular recognition interactions between functionalism CNT and target analytes.
For instance, researchers have developed flexible hydrogen sensors using single-walled carbon nanotubes decorated with palladium nanoparticles.
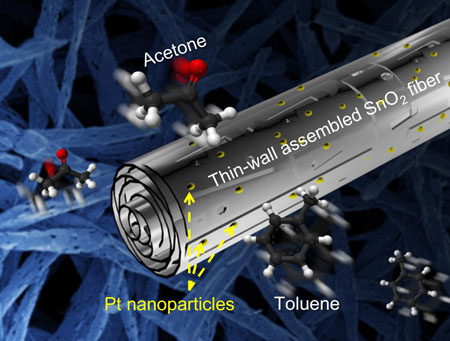
Nanowires and nanofibers have also been used to build chemiresistive sensors for the diagnosis of diseases. They have been used to maximize gas sensor responses in exhaled breath analysis for the detection of volatile organic compounds (which are biomarkers for various diseases; for example, acetone, hydrogen sulfide, ammonia, and toluene can be used as biomarkers for evaluating diabetes, halitosis, kidney malfunction, and lung cancer, respectively).
In one example, porous tin oxide (SnO2) nanofibers have been demonstrated to detect acetone at levels of around 0.1 ppm, which is eight times lower than the required gas-sensing level for diagnosing diabetes.
Nanosensors based on graphene
Another carbon nanomaterial, functionalized graphene, holds exceptional promise for biological and chemical sensors. Already, researchers have shown that the distinctive 2D structure of graphene oxide (GO), combined with its superpermeability to water molecules, leads to sensing devices with an unprecedented speed ("Ultrafast graphene sensor monitors your breath while you speak").
Scientists have found that chemical vapors change the noise spectra of graphene transistors, allowing them to perform selective gas sensing for many vapors with a single device made of pristine graphene – no functionalization of the graphene surface required ("Selective gas sensing with pristine graphene").
Researchers also have begun to work with graphene foams – three-dimensional structures of interconnected graphene sheets with extremely high conductivity. These structures are very promising as gas sensors ("Graphene foam detects explosives, emissions better than today's gas sensors") and as biosensors to detect diseases (see for instance: "Nanotechnology biosensor to detect biomarkers for Parkinson's disease").
Nanosensors based on bulk nanostructured materials
Whereas several properties of nanoparticles are useful for applications in nanosensors, their catalytic behavior is one of the most important with regard to electrochemical sensing devices. For instance, platinum nanoparticles supported on materials such as porous carbon or noble metals such as gold are reported to be relevant in the design of gas diffusion electrodes.
Another property, their high surface area, makes nanoparticles suitable for immobilizing molecules, polymers or biomaterial coatings that allow the generation of composite materials with tunable surface properties. For example, modifying metal nanoparticles with pre designed receptor units and assembling them on surfaces could lead to new electrochemical sensors with tailored specificities.
Simple and highly-selective electroanalytical procedures can also be achieved by proper functionalization of nanoparticles. Finally, stable nanoparticles can substitute amplifying labels of limited stability, such as enzymes or liposomes, with equivalent or improved sensitivities.
Nanosensors based on metal-organic frameworks (MOFs)
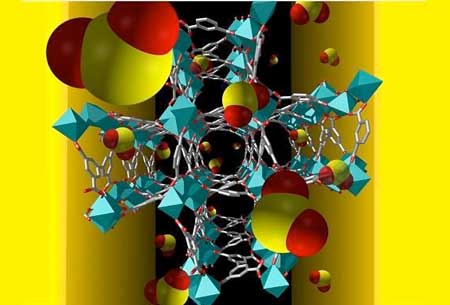
Metal-organic frameworks (MOFs) are organic-inorganic hybrid crystalline porous materials that consist of a regular array of positively charged metal ions surrounded by organic 'linker' molecules. The metal ions form nodes that bind the arms of the linkers together to form a repeating, cage-like structure. Due to this hollow structure, MOFs have an extraordinarily large internal surface area, which makes them ideal materials for gas sensing.
By making the MOF from different metal atoms and organic linkers, researchers can create materials that selectively absorb specific gases into tailor-made pockets within the structure.
One example is a thin-film a tailor-made MOF, coated onto an electrode, that forms an electronic sensor that could detect traces of sulfur dioxide gas.
Other examples of nanosensor applications
A simple single nanoscale sensor and light spectroscopy (fluorescence) can be used for sensing the difference in potential across a biological membrane such as a cell wall. This non-contact, optical voltage nanosensor is based on a F??rster resonance energy transfer (FRET) sensor on a DNA origami.
A graphene nanosensor enables real-time monitoring of insulin at physiologically relevant concentrations – down to a concentration of approximately 35 pM. This devices employs an aptamer-based graphene field-effect transistor (GFET) nanosensor that can rapidly respond to external stimuli such as the binding between surface-immobilized aptamer molecules with insulin. This results in significant changes of the electrostatic charge characteristics in the close proximity of the graphene surface.
A gel-based sensor technology can help medical physicists and oncologists effectively plan radiotherapy in the clinic, reduce accidental overexposures, and reduce radiation-induced toxicity. In contrast to traditional inflexible sensors, these plasmonic nanosensor gels indicate exposure levels as radiation delivered to the hydrogel increases the intensity of color developed in the gel.
By interfacing passive, wireless graphene nanosensors onto biomaterials via silk bioresorption, researchers have developed graphene nanosensor tattoos on teeth in order to monitor harmful oral bacteria.
A great example of nanosensor technology is the ability of chips with molecular probes to detect intracellular biological parameters, i.e. from within a living cell.
