IR Spectroscopy: A Powerful Tool for Material Characterization
What is IR Spectroscopy?
IR spectroscopy, or infrared spectroscopy, is an analytical technique used to identify and study chemical substances based on their interaction with infrared radiation. It measures the absorption of infrared light by a sample at different wavelengths to produce a spectrum that provides information about the sample's molecular structure and composition.
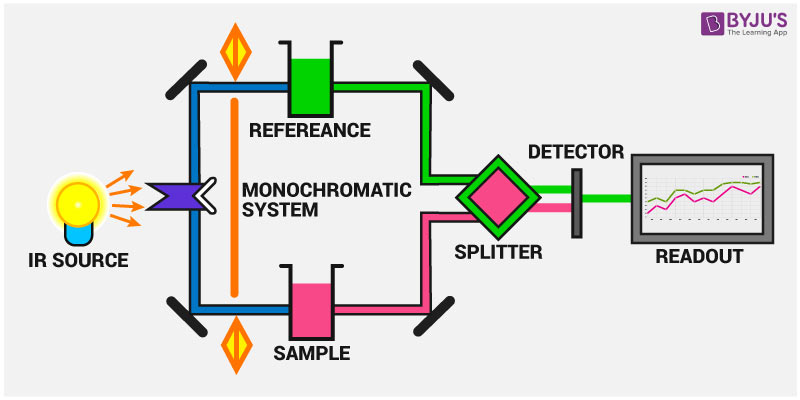
Principles of IR Spectroscopy
IR spectroscopy relies on the fact that molecules absorb specific frequencies of infrared light that correspond to the vibrational frequencies of their chemical bonds. When a molecule absorbs infrared radiation, its chemical bonds vibrate, causing a change in the molecule's dipole moment. The resulting absorption spectrum is unique to each molecule and provides a "fingerprint" that can be used for identification.
Types of Molecular Vibrations
There are two main types of molecular vibrations that are relevant to IR spectroscopy:
- Stretching Vibrations: These involve the change in the distance along the axis of a bond between two atoms. Stretching vibrations can be symmetric (both atoms move in the same direction) or asymmetric (atoms move in opposite directions).
- Bending Vibrations: These involve the change in the angle between two bonds. Bending vibrations can be in-plane (scissoring, rocking) or out-of-plane (wagging, twisting).
Each type of vibration occurs at a specific frequency, which depends on the mass of the atoms involved and the strength of the chemical bond.
Instrumentation
A typical IR spectrometer consists of four main components:
- IR Source: Generates a broad spectrum of infrared light.
- Interferometer: Splits the light beam into two and creates an interference pattern.
- Sample Compartment: Holds the sample and allows the infrared light to pass through it.
- Detector: Measures the intensity of the infrared light that passes through the sample.
The most common type of IR spectrometer is the Fourier Transform Infrared (FTIR) spectrometer, which uses an interferometer to collect high-spectral-resolution data over a wide spectral range.
Sample Preparation and Measurement
Sample preparation is a critical step in IR spectroscopy. Samples can be analyzed in various forms, such as gases, liquids, or solids. Solid samples are typically prepared by grinding the sample with potassium bromide (KBr) and pressing it into a thin pellet. Liquid samples can be analyzed using a liquid cell with IR-transparent windows, while gases are measured using a gas cell.
Once the sample is prepared, it is placed in the sample compartment of the IR spectrometer. The instrument then measures the intensity of the infrared light that passes through the sample at different wavelengths, producing an absorption spectrum.
Data Interpretation
The absorption spectrum obtained from an IR spectroscopy measurement provides valuable information about the molecular structure of the sample. The position, shape, and intensity of the absorption bands can be used to identify functional groups and elucidate the chemical composition of the sample.
Interpretation of IR spectra involves comparing the observed absorption bands with reference spectra of known compounds. Correlation charts and spectral libraries are used to aid in the identification of functional groups and the elucidation of molecular structures.
Applications in Nanotechnology
IR spectroscopy is widely used in nanotechnology for the characterization of nanomaterials and the study of surface chemistry at the nanoscale. Some key applications include:
- Identification of functional groups on nanoparticle surfaces.
- Monitoring chemical reactions and surface modifications of nanomaterials.
- Studying the adsorption and desorption of molecules on nanostructured surfaces.
- Investigating the interactions between nanomaterials and biological systems.
- Quality control and purity assessment of nanomaterials.
IR spectroscopy is often used in combination with other characterization techniques, such as Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), and transmission electron microscopy (TEM), to provide a comprehensive understanding of the properties and behavior of nanomaterials.
Challenges and Future Perspectives
Despite its widespread use, IR spectroscopy faces several challenges when applied to nanomaterials. One of the main challenges is the low signal-to-noise ratio due to the small sample size and the presence of interfering signals from the substrate or environment. Advanced techniques, such as surface-enhanced infrared absorption (SEIRA) spectroscopy and tip-enhanced Raman spectroscopy (TERS), have been developed to overcome these limitations and improve the sensitivity and spatial resolution of IR spectroscopy at the nanoscale.
Future developments in IR spectroscopy for nanotechnology will focus on the integration of advanced optical techniques, such as near-field scanning optical microscopy (NSOM) and plasmonic nanoantennas, to enable high-resolution chemical imaging and single-molecule detection. The combination of IR spectroscopy with machine learning and data analytics will also play a crucial role in accelerating the discovery and optimization of novel nanomaterials and devices.
