Scanning Ion Conductance Microscopy (SICM): Nanoscale Imaging of Living Systems
What is Scanning Ion Conductance Microscopy?
Scanning Ion Conductance Microscopy (SICM) is a non-contact scanning probe microscopy technique that enables high-resolution imaging of living cells and tissues at the nanoscale. SICM measures the ion current flowing through a small glass pipette to map the topography and conductivity of biological samples in a liquid environment, providing insights into cellular structures and functions without causing damage.
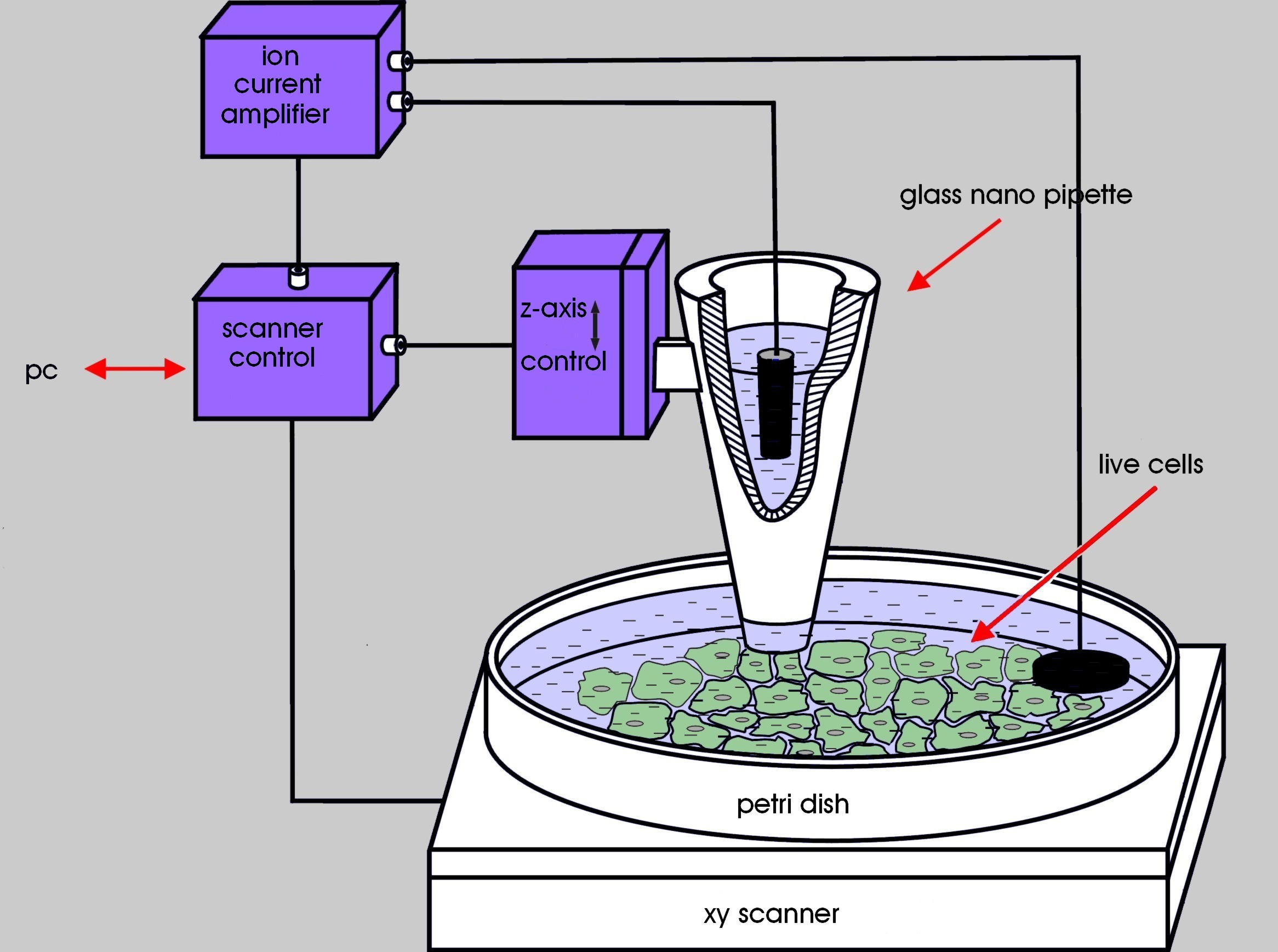
Principles of SICM
SICM is based on the following key principles:
- Ion Current Measurement: A glass pipette filled with an electrolyte solution is used as a probe. As the pipette approaches the sample surface, the ion current flowing through the pipette decreases due to the increased resistance. This change in ion current is used to detect the sample surface and maintain a constant distance between the pipette and the sample.
- Feedback Control: SICM employs a feedback control system to maintain a constant pipette-sample distance. The system adjusts the pipette position in real-time based on the measured ion current, ensuring that the pipette does not contact the sample surface, thus preventing damage to delicate biological structures.
- Scanning and Imaging: The pipette is scanned over the sample surface in a raster pattern, and the ion current is recorded at each point. The collected data is then used to reconstruct a high-resolution topographical image of the sample surface, revealing nanoscale features and structures.
Advantages of SICM
SICM offers several advantages over other scanning probe microscopy techniques for imaging biological samples:
- Non-Contact Imaging: SICM allows for non-contact imaging of delicate biological samples, minimizing the risk of damage or alteration to the sample during the imaging process. This is particularly important for studying living cells and tissues in their native state.
- High Resolution: SICM can achieve nanoscale resolution, typically in the range of 20-50 nm, depending on the pipette size and imaging conditions. This high resolution enables the visualization of fine cellular structures, such as membrane proteins, cytoskeletal elements, and organelles.
- Liquid Environment: SICM operates in a liquid environment, making it suitable for imaging living biological samples under physiological conditions. This allows for the study of dynamic cellular processes, such as cell migration, membrane dynamics, and ion channel activity.
- Functional Imaging: In addition to topographical imaging, SICM can also provide functional information about the sample. By measuring the local ion conductivity, SICM can map the distribution of ion channels and transporters on the cell surface, providing insights into cellular physiology and signaling.
Comparative Analysis with Other Microscopy Techniques
SICM offers unique advantages over other microscopy techniques for imaging living biological systems:
Atomic Force Microscopy (AFM)
While Atomic Force Microscopy provides high-resolution topographical imaging, it requires physical contact between the probe and the sample, which can cause damage to delicate biological structures. SICM, on the other hand, enables non-contact imaging, making it more suitable for studying living cells and tissues. Additionally, SICM can operate in a liquid environment, allowing for the imaging of samples under physiological conditions, which is challenging with conventional AFM.
Electron Microscopy (EM)
Electron microscopy techniques, such as Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM), offer high resolution imaging but require sample preparation steps that can alter the native state of biological samples. These techniques typically involve fixation, dehydration, and coating of the sample, which can introduce artifacts and limit the study of dynamic processes. SICM allows for the imaging of living samples in their native state, providing a more accurate representation of cellular structures and functions.
Optical Microscopy
Optical microscopy techniques, such as Confocal Microscopy and Super-Resolution Microscopy, provide valuable insights into cellular structures and dynamics. However, these techniques are limited by the diffraction limit of light, which restricts their resolution to around 200 nm. SICM can achieve higher resolution, down to 20-50 nm, enabling the visualization of finer cellular details. Moreover, SICM provides direct topographical information, which is not readily available with optical microscopy techniques.
In summary, SICM is preferred over other microscopy techniques when non-contact, high-resolution imaging of living biological samples in their native environment is required. Its ability to operate in liquid, maintain sample integrity, and provide both topographical and functional information makes it a valuable tool for studying cellular structures and processes at the nanoscale.
Applications of SICM
SICM has found numerous applications in the study of biological systems at the nanoscale:
Cell Surface Imaging
SICM is widely used for high-resolution imaging of the cell surface topography. It can reveal intricate details of the plasma membrane, such as microvilli, filopodia, and membrane invaginations. This information is valuable for understanding cell morphology, adhesion, and communication.
Ion Channel Mapping
SICM can be used to map the distribution and activity of ion channels on the cell surface. By measuring the local ion conductivity, SICM can identify the location and density of specific ion channels, such as potassium, sodium, or calcium channels. This technique has been applied to study the role of ion channels in various physiological processes, including neurotransmission, muscle contraction, and cell signaling.
Tissue Imaging
SICM has been extended to image biological tissues, such as epithelial layers, blood vessels, and neural networks. It can provide high-resolution images of the tissue surface, revealing the organization and interactions of individual cells within the tissue. This information is valuable for understanding tissue structure, function, and pathology.
Drug Delivery and Nanoinjection
SICM can be combined with other techniques, such as pressure injection or electroporation, to deliver drugs, biomolecules, or nanoparticles to specific cells or subcellular compartments. The precise positioning capability of SICM allows for targeted delivery with minimal damage to the surrounding cells or tissues.
Challenges and Future Perspectives
Despite the significant advances in SICM technology, several challenges remain. One of the main limitations is the relatively slow imaging speed compared to other scanning probe microscopy techniques. This is due to the need for precise feedback control and the inherent time required for ion current measurements. Efforts are being made to develop faster SICM systems, such as using multiple pipettes in parallel or implementing advanced feedback control algorithms.
Another challenge is the complexity of data interpretation, particularly when imaging heterogeneous biological samples. The ion current signal can be influenced by various factors, such as sample conductivity, surface charge, and pipette geometry. Developing robust data analysis methods and combining SICM with complementary techniques, such as fluorescence microscopy or electrophysiology, can help overcome these challenges and provide a more comprehensive understanding of the sample.
Future developments in SICM are expected to focus on improving the imaging speed, resolution, and functionality. The integration of SICM with other imaging modalities, such as super-resolution microscopy or atomic force microscopy, can provide multi-dimensional information about biological systems. Additionally, the development of novel pipette designs and functionalized probes can expand the capabilities of SICM, enabling the measurement of specific molecular interactions or the detection of biochemical signals at the nanoscale.
Further Reading
Chemical Reviews, Scanning ion conductance microscopy
ChemElectroChem, Mapping Surface Charge of Individual Microdomains with Scanning Ion Conductance Microscopy
MedComm – Biomaterials and Applications, Applications of scanning ion conductance microscope in biomedical fields
