| Jan 19, 2012 |
Controlling molecular self-assembly via different pathways
|
|
(Nanowerk News) Researchers at Eindhoven University of Technology (TU/e) have succeeded in monitoring and controlling a molecular self-assembly process via different pathways. While it was formerly thought that the molecules form the right structure by themselves, this research shows that the assembly process can follow different pathways yielding different structures; in this case polymer chains with left- and right-handed helical directions. This new knowledge is of great importance for the understanding of supramolecular polymers, in which small differences in the way the molecular building blocks are organized can have a large influence on the properties of the resulting material.
|
|
The results were published online by Nature yesterday ("Pathway complexity in supramolecular polymerization").
|
|
Molecular self-assembly is the process through which molecular building blocks organize themselves into supramolecular structures. This occurs frequently in nature, for example in the formation of cell membranes. Controlling the principles of molecular self-assembly opens the way to totally new materials with special properties, for example self-repairing coatings. The properties of these materials are strongly influenced by the way the building blocks are assembled; a small difference in their organization can lead to very different properties. This makes an in depth understanding of the assembly process, and the ability to manipulate it in subtle ways, very important.
|
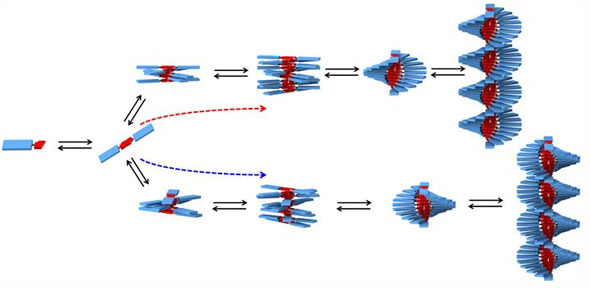 |
| A diagram showing the formation of helical SOPV polymer chains.
|
|
In their experiments, Korevaar and his fellow researchers use a molecular building block that can be studied in detail using spectroscopy: S-chiral oligo(p-phenylenevinylene) or SOPV. Molecules of this kind are frequently used in organic electronic devices, in which small differences in the morphology of the material lead to large differences in their properties. At the start of the assembly process SOPV first forms unstructured clusters, which subsequently grow into neatly organized left-handed 'spiral staircase' like helical structures.
|
|
Up to now it was assumed that the self-assembly of a molecule can lead to only one single end-product, and that the intermediate process steps are not important and take place too rapidly to even allow them to be studied. This now appears to be incorrect: in fact the intermediate process steps are very important, and lead to different variants. For example if the assembly process of SOPV takes place rapidly, 'spiral staircase' structures with opposite helical direction are formed. Temporary addition of tartaric acid, a small molecule that attaches itself to the SOPV molecules, forces the assembly process completely towards this alternative form. Detailed studies show that the two different helical structures compete for the available molecules as building blocks.
|
|
"This knowledge has significant impact on an optimal self-assembly process, and we can now use it for more applied supramolecular systems which are much more difficult to study", says Peter Korevaar, first author of the Nature publication.
|
|
Korevaar is carrying out his research at the Institute for Complex Molecular Systems (ICMS) at TU/e. The ICMS is an institute that brings together excellent researchers from a range of disciplines to address the question: how far can we push chemical self-assembly?
|

