| Posted: Apr 25, 2017 | |
Defects in graphene enable selective permeability of gases |
|
| (Nanowerk Spotlight) Future electric vehicle technologies literally need a fresh breath of air. Li-O2 batteries, which store and deliver energy much higher than the present Li-ion technologies by drawing oxygen from air, are touted as the panacea for powering electric vehicles (read more: "New lithium-oxygen battery greatly improves energy efficiency, longevity"). | |
| Despite their potential, the practical use of Li-O2 batteries is seriously limited by the corrosion of Li metal by ambient water vapor from air. | |
| One way to circumvent this issue is to use an oxygen selective membrane that allows only oxygen into the battery while stopping or slowing water vapor intake. The membrane must be mechanically robust and yet sufficiently thin and light so as to not increase deadweight of the battery. | |
| Researchers from the Clemson Nanomaterials Institute and the Ural Federal University (Russia) have collaboratively discovered a way to make the thinnest possible oxygen selective membrane using graphene. Their results have been published in Nanoscale ("Influence of dopants on the impermeability of graphene"). | |
 |
|
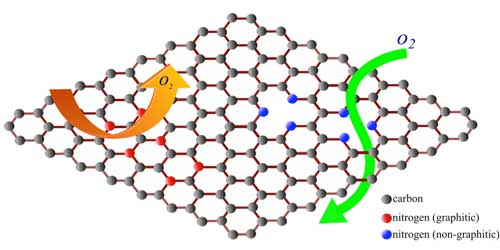
| Defects are often written off in materials as performance limiters. Researchers from the Clemson Nanomaterials Institute and the Ural Federal University (Russia) identified specific defect configuration that could be used for tailoring the permeability of graphene. Their work showed that nitrogen dopants can be used to turn otherwise impermeable graphene into an oxygen selective membrane. (Image: Clemson University) | |
| "In its pristine/perfect atomic configuration, graphene is impermeable to all gases (read more: "World's thinnest balloon made of graphene". But, pores in graphene can selectively allow the transport of gas/water molecules," says Sai Sunil Mallineni, a PhD candidate from Clemson Nanomaterials Institute and the lead author on the Nanoscale paper. "We developed an in situ technique to induce pores in graphene by doping it with nitrogen during the growth process." | |
| Graphene, an atom-thick layer of carbon, chemically grown on a copper foil protects it from oxidation by forming an impermeable barrier. | |
| Doping the graphene sheet with nitrogen inevitably breaks some carbon bonds (see the figure above) in graphene opening nanoscopic pores. | |
| The Clemson and Ural team observed that such pores in doped graphene selectively allow oxygen leading to oxidation of the underlying copper foil unlike pristine graphene. | |
| "Conventional protective membrane coatings are usually few microns thick. On the other hand, graphene coatings are extremely thin and yet robust," notes Dr. Ramakrishna Podila, Assistant Professor of Physics at Clemson and a co-author on this project. "We hope to enable new applications such as Li-O2 batteries using graphene-based oxygen selective membranes." | |
| "We ultimately wish to induce pores of different configurations to selectively filter ions, molecules and gases," hopes Dr. Sriparna Bhattacharya, Research Assistant Professor at CNI who lead this project. "To this end, we are now exploring other dopants such as boron for in situ engineering of the pores to filter specific gases." | |
| Provided by Clemson University as a Nanowerk exclusive | |
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
