| Posted: Jun 16, 2015 |
The perils of platinum
|
|
(Nanowerk News) Visions of dazzling engagement rings may pop to mind when platinum is mentioned, but a significant share of the nearly half a million pounds of the rare metalExternal link mined each year ends up in vehicle emission systems and chemical manufacturing plants. The silvery white metal speeds up or enhances reactions, a role scientists call serving as a catalyst, and platinum is fast and efficient performing this function.
|
|
Because of its outstanding performance as a catalyst, platinum plays a major role in fuel cells. Inside a fuel cell, tiny platinum particles break apart hydrogen fuel to create electricity. Leftover protons are combined with oxygen ions to create pure water.
|
|
Fuel cells could let scientists turn wind into fuel. Right now, electricity generated by wind turbines is not stored. If that energy could be converted into hydrogen to power fuel cells, it would turn a sporadic source into a continuous one.
|
|
The problem is the platinum – a scarce and costly metal. Scientists funded by the U.S. Department of Energy's Office of Science are seeing if something more readily available, such as iron or nickel, could catalyze the reaction.
|
|
But, earth-abundant metals cannot simply be used in place of platinum and other rare metals. Each metal works differently at the atomic level. It takes basic research to understand the interactions and use that knowledge to create the right catalysts.
|
|
At the Center for Molecular Electrocatalysis, an Energy Frontier Research Center, scientists are gaining new understanding of catalysts based on common metals and how they move protons, the positively charged, oft-ignored counterpart to the electron.
|
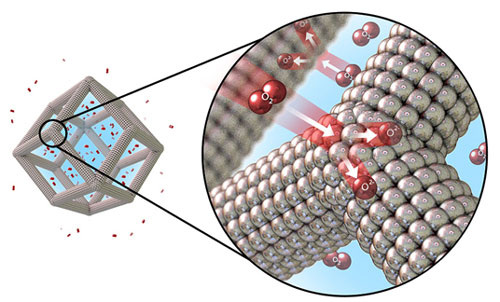 |
| A team of scientists designed platinum-nickel nanoframes with multilayered platinum skin structure. The structure catalyzes the oxygen reduction reaction (shown here) on the surface. (Image courtesy of Argonne National Laboratory and Lawrence Berkeley National Laboratory)
|
|
Center Director Morris Bullock and his colleagues showed that protons’ ability to move through the catalyst greatly influences the catalyst's speed and efficiency. Protons move via relays -- clusters of atoms that convey protons to or from the active site of catalysts, where the reaction of interest occurs. The constitution, placement, and number of relays can let a reaction zip along or grind to a halt. Bullock and his colleagues are creating "design guidelines" for building relays.
|
|
Further, the team is expanding the guidelines to examine proton movement related to the solutions and surfaces where the catalyst resides. For example, matching the proton-donating abilityExternal link of a nickel-based catalyst to that of the surrounding liquid, much like matching your clothing choice with the event you’re attending, eases protons’ travels. The benefit? Speed. A coordinated catalyst pumped out 96,000 hydrogen molecules a second -- compared to just 27,000 molecules a second without the adjustment.
|
|
This and other research at the Energy Frontier Research Center is funded by the DOE Office of Science’s Office of Basic Energy Sciences. The Center is led by Pacific Northwest National Laboratory.
|
|
At two other labs, research shows how changing the catalyst’s superstructure, which contains the proton relays and wraps around the active site, can also increase the speed of the reaction. Led by Argonne National Lab’s Vojislav Stamenkovic and Berkeley Lab’s Peidong Yang, researchers created hollow platinum and nickel nanoparticles, a thousand times smaller in diameter than a human hair. The 12-sided particles split oxygen molecules into charged oxygen ions, a reaction that’s needed in fuel cells. The new catalyst is far more active and uses far less platinum than conventional platinum-carbon catalysts (Science, "Highly Crystalline Multimetallic Nanoframes with Three-Dimensional Electrocatalytic Surfaces").
|
|
Building the catalysts begins with tiny structures made of platinum and nickel held in solution. Oxygen from the air dissolves into the liquid and selectively etches away some of the nickel atoms. The result is a hollow framework with a highly active platinum skin over the surface. The open design of the catalyst allows the oxygen to easily access the platinum. The new catalyst has a 36-fold increase in activity compared to traditional platinum–carbon catalysts. Further, the new hollow structure continues to work far longer in operating fuel cells than traditional catalysts.
|
|
These and other projects funded by the Energy Department answer questions vital to creating fuel cells that use platinum more efficiently and one day will lead to nickel, iron, or other common metals working in fuel cells to power cars, computers, and skyscrapers, while platinum rests, sparkling, in the wedding band section of jewelers' cases.
|

