| Posted: Dec 02, 2015 |
A quantum spin on molecular computers
(Nanowerk News) If quantum computers existed, they would revolutionize computing as we know it. Based on fundamental properties of matter, the potential power of these theoretical workhorses would solve problems in a new way, cracking extremely complex spy codes and precisely modeling chemical systems in a snap. This week in ACS Central Science ("Millisecond Coherence Time in a Tunable Molecular Electronic Spin Qubit"), researchers create cleverly designed molecules to get one step closer to this goal.
|
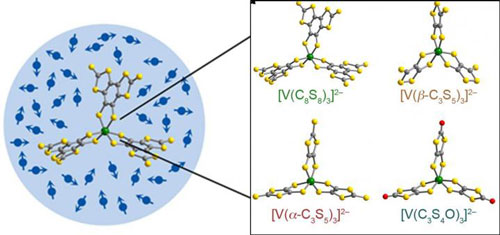 |
| One day powerful quantum computers could be made from molecules like these -- vanadium complexes. (© American Chemical Society)
|
|
Traditional computers rely on transistors that occupy one of two states -- that's what those archetypal zeroes and ones refer to, and each digit is a "bit." Quantum computing would use three states, improving its information storage capacity exponentially. Whereas a small app like "Angry Birds" takes up about 40,000 standard bits, a computer made with just 1,000 quantum bits, or "qubits," could easily and quickly break modern encryption schemes or more precisely model how a pharmaceutical drug candidate would perform in a person.
|
|
The biggest challenge of quantum computing, however, is making the qubit. Some of the most promising qubits today use electrons, specifically their "spin" state. Spin can have two states, just like a bit, but also a combination of both to form a third state, called "superposition." But very few molecules stay in the superposition state long enough to measure, which makes them difficult to use in computing. One reason is that the interaction of spins on most nuclei can interfere with the electronic ones.
|
|
To get closer to a real, functional qubit, Danna Freedman and colleagues turned to metal complexes, where most of those problematic nuclear spins were eliminated.
|
|
Freedman and colleagues synthesized vanadium complexes with arms made of carbon and sulfur. As long as the system was kept cold, these molecules kept superposition longer than any metal complexes previously reported. They also kept that state for just as long as other bulk materials currently under consideration. These new molecules show that under the right conditions, inorganic complexes can function as viable qubits. In addition, the complexes may prove to be superior to other potential materials because their defined chemical structure could more easily allow the organized design of functional devices.
|
|
To get a little meta: it's possible that one day computers made of just a handful of small molecules will be used to make predictions about other molecules.
|

