| Posted: Apr 18, 2011 | |
Self-sintering conductive inks simplify printing of plastic electronics |
|
| (Nanowerk Spotlight) Ink-jet printing of metal nanoparticles for conductive metal patterns has attracted great interest as an alternative to expensive fabrication techniques like vapor deposition. The bulk of the research in this area focuses on printing metal nanoparticle suspensions (metallic ink) for metallization. For example, silver and gold nanoparticle suspensions have been inkjet printed to build active microelectromechanical systems (MEMS), flexible conductors and radio frequency identification (RFID) tags. Nobel metals like silver and gold are preferred nanoparticles for ink-jet formulations because they are good electrical conductors and they do not cause oxidation problems. | |
| These conductive inks require a post printing step though. Printing conductive features by metallic nanoparticle inks must be followed by an additional step of sintering, usually achieved by heating to elevated temperatures. In this step, the nanoparticles composing the pattern will coalesce to form a continuous electrical contact. | |
| In new work, researchers have now demonstrated a new conductive ink that won't require a post printing sintering step. It is achieved by the addition of a latent sintering agent that gets into action after the printing step. Once the solvent evaporates, the sintering agent concentration increases, leading to the spontaneous sintering of the nanoparticles. | |
| "The idea to form a self-sintering conductive ink was not published previously" Shlomo Magdassi from the Institute of Chemistry at the Hebrew University of Jerusalem, tells Nanowerk. "Therefore, our motivation was to develop a technique that allows us to print conductive patterns in a single step, without heating to elevated temperatures. This is of outmost importance when fabricating plastic electronics devices." | |
| As they reported in a recent paper in ACS Nano ("Conductive Inks with a "Built-In" Mechanism That Enables Sintering at Room Temperature"), the team achieved a new 'self-sintered' metal dispersion in which the sintering is triggered by changes in concentration of chloride ions. | |
 |
|
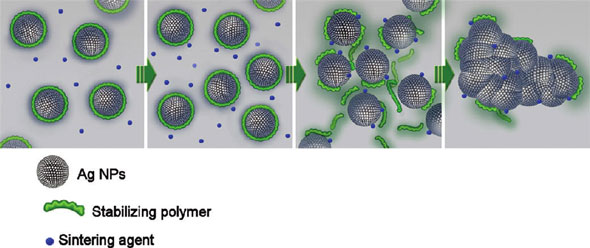
| Schematic illustration of the stabilizer detachment, which leads to the nanoparticle sintering (the green lines represent the polymeric stabilizer; the blue spheres represent the sintering agent). (Reprinted with permission from American Chemical Society) | |
| "Instead of carrying out the sintering as an additional sequential step after printing the metallic nanoparticles, we developed a new dispersion that can self-sinter spontaneously once it dries on the substrate," explains Michael Grouchko, a PhD student in Magdassi's group and the paper's first author. "This dispersion is mainly a dispersion of electrosterically stabilized silver nanoparticles, together with a low concentration of a destabilizer, which acts as a sintering agent and comes into action only upon drying of the dispersion." | |
| Magdassi further explains that the sintering agent, which can be a simple electrolyte such as sodium chloride, destabilizes the silver nanoparticles and leads to their close contact. "The chloride ions replace and detach the anchoring groups of the polymeric stabilizer from the nanoparticles' surface and thus enable their coalescence and sintering." | |
| The stability of the silver dispersion at low sodium chloride concentration (<50 mM) enabled the team to test their self-sintered dispersion as a conductive inkjet ink – the sintering takes place at room temperature during the water evaporation. | |
| The team notes that the conductivity of the self-sintered silver pattern was only about 10% that of bulk silver. This is due to the well-sintered particles, but could be improved if a denser packing of the nanoparticles was obtained. | |
| "The low-density packing of the nanoparticles is a result of the immediate coalescence of the nanoparticles in the liquid, which does not enable any optimization of the packing" says Grouchko. "In order to achieve denser packing but still use the same destabilization process (with Cl-ions), the two processes, destabilization and packing of the particles, should be separated." | |
| That means that in a first step, a dense structure should be obtained due to evaporation of the water, and then the closely packed nanoparticles should be sintered. | |
| Since treatment of a dried silver nanoparticle array by a sodium chloride solution will lead to destruction of the obtained packing, the researchers overcame this problem by using a different source of Cl-ions – hydrogen chloride vapors – which do not destroy the dense packing. | |
| Working with a more densely packed layer (which was obtained from a dispersion containing 20 wt % silver), they found that the silver network obtained after 10 seconds exposure to hydrogen chloride vapors is obviously much denser and has more percolation paths than the one obtained by sodium chloride. | |
| "The metallic nanoparticles actually behave as soft material" says Grouchko. "This approach leads to very high conductivities, up to 41% of the conductivity of bulk silver, the highest reported conductivity of structures obtained at room temperature." | |
| Magdassi points out that, since this new concept doesn't require heating the printed patterns to elevated temperatures, it paves the way to the formation of conductive patterns on sensitive substrates like plastic and paper. | |
| "We believe that the same concept can be applied to low cost metallic particles such as copper, a subject that we are already working on with good results" he adds. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
