| Oct 23, 2020 | |
Advanced cantilever-based techniques for virus research |
|
| (Nanowerk Spotlight – Application Note) During the last century, humans have endured multiple severe viral outbreaks including the Spanish flu and other influenza epidemics, HIV, SARS-CoV, MERS, Ebola and the recent SARS-CoV2. During this period, science has made strides in understanding many different facets of virus outbreaks including viral infectivity, pathogenicity, and virulence, transmission, and propensity to cause an epidemic/pandemic. | |
| Modern techniques that include cell culture, infectivity assays, polymerase chain reactions, fluorescence microscopy, electron microscopy, and immunoassays have helped us gain a better understanding about the virus life cycle, viral replication, and the virus-host interaction. We now have a better grasp on the structure and function of the viral particle, viral genome, RNA or DNA expression levels, virus-host cell interaction and immune response. These advances have resulted in the development of vaccines and drugs to treat several different viruses. | |
| Atomic force microscopy (AFM) is one of the newer techniques available for virus research. AFM is a cantilever-based technique that utilizes a sharp tip to interrogate surfaces at resolutions well below the optical diffraction limit. Beyond imaging, AFM is also a powerful tool for nano-mechanical probing and nano-manipulation. | |
| One of the primary advantages of AFM is that it can operate on samples immersed in liquid. This empowers experiments on living cells at physiologically relevant conditions. AFM imaging has been used to study live virus particles, to investigate the dimension and morphology and packaging of viral genomic material. Beyond imaging, AFM has been used for manipulation of single viruses by force spectroscopy to study early events of virus-host interactions. | |
| In this article we summarize two relatively new cantilever-based approaches for the study of virus-host interactions at the single cell level. In the first example, virus particles were deposited on a single cell using a hollow cantilever and the host cell response upon virus infection was studied. In the second example, single cells bound to a cantilever were infected with a virus and effect of infection on the cell mass was monitored over several hours. | |
Cooperative Vaccinia Infection |
|
| The first study aimed to study the cooperativity of virus particles in cell infection. In other words, to find out how the probability of virus infection depends on the amount of virus particles attacking a cell. To study cooperativity of virus infection, accurate control is needed on the timing of infection and the number of virions to which a cell is exposed. | |
| Two pioneering labs, the Laboratory of Biosensors and Bioelectronics and the Institute of Microbiology of the ETH Zurich used the FluidFM® to precisely deliver a defined number of virus particles onto single cells. | |
 |
|
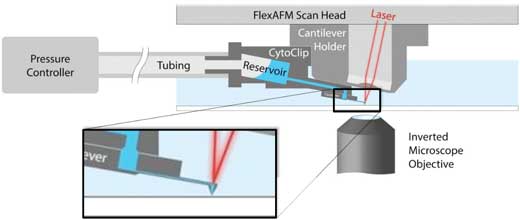
| Figure 1. Schematic of AFM with FluidFM. A hollow cantilever featuring an opening at its free end is filled with liquid from a reservoir. By applying a positive or negative pressure liquid can be secreted out of or aspired into the cantilever to locally manipulate a sample. The AFM handles accurate force control and the complete system is integrated on an inverted, optical microscope to provide optical access to the sample. (click on image to enlarge) | |
| FluidFM is a cantilever-based manipulation tool that can be added to an AFM and uses hollow cantilevers to locally deposit or aspire material (1). A schematic of the FluidFM technology is presented in Figure 1. | |
| In this study an AFM with FluidFM® functionality was integrated with a fluorescence microscope to study the single cell response after exposure to different numbers of virus particles. The research groups at ETH developed a protocol to use FluidFM to deposit 1-12 mature vaccinia virions on single cells (2). The virus deposition process is described in Figure 2. | |
 |
|
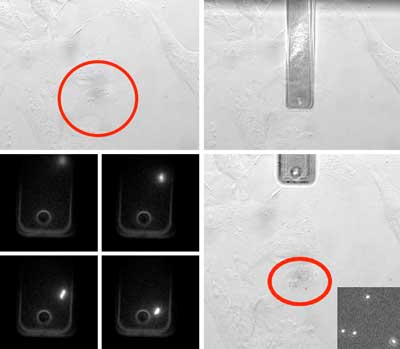
| Figure 2. Four phases of virus deposition with a hollow cantilever to study single cell infection. I. A cell is selected using the optical microscope. II. The hollow cantilever is moved over the cell and brought into gentle contact under force control. III. During deposition the virions exiting the cantilever are monitored. IV. The number of virions on the cell membrane is counted. (click on image to enlarge) | |
| To ensure that the virus particles did not leak into the solution and cause uncontrolled infection, the FluidFM hollow cantilever was gently pushed on to the cell forming a seal between the cantilever and the cell surface. Since the AFM provides sub-nanonewton force control, the cells are not damaged during this process. | |
| To follow the deposition of virions and the progress of infection, the researchers used a recombinant vaccinia virus (VACV), that incorporated the fluorescent protein mCherry into the virus core protein A5. This fusion protein allowed tracing of assembled virion particles by fluorescence. This allowed the number of deposited particles to be counted and assembly of new virions in the late infection phase to be monitored. | |
| The virus also encoded for eGFP under control of an early/late viral promoter. The eGFP was expressed after entry into host cells creating a reporter of the different infection phases (Figure 3). | |
 |
|
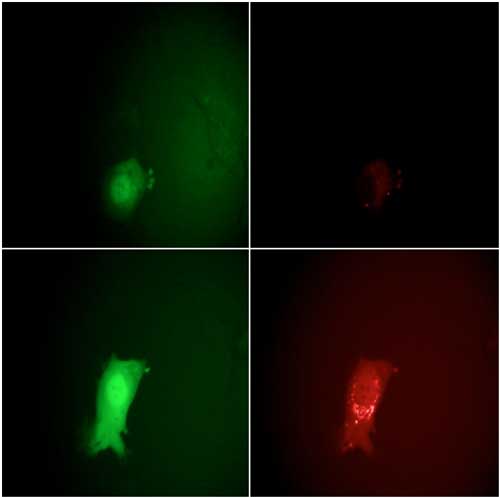
| Figure 3. Monitoring the stages of the VACV lifecycle microscopically by eGFP and mCherry fluorescence signals. Top row: 7 hours post infection, bottom row: 11.5 hours. The intensity of the EGFP signals on the left clearly show the entering of the early and late viral gene expression phases. The strong increase in the mCherry intensity at the right between 7 and 11.5 hours indicates the assembly of new virions. | |
| The results of this study show cooperativity of the virus infection. A cell only had about 10% chance of being infected when targeted by a single virus (n=73). This probability increased to about 35% when attacked with 2 particles (n=23) and to 90% when exposed to 4 virus particles (n=20). Possible reason for this cooperativity is that the host cells antiviral mechanism is weakened when multiple viruses arrive simultaneously. | |
Effect of virus infection on cell mass |
|
| In 2017 researchers from the Biophysics lab of the ETH Zurich presented an inertial pico-balance to non-invasively measure the mass of adherent cells at high mass and time resolution(3). The pico-balance has picogram mass sensitivity and millisecond time resolution. The mass is derived from the resonance frequency of a cantilever, which is oscillated with sub-nanometer amplitudes using a laser with fluctuating intensity focused at the base of the cantilever. The resonance frequency of the cantilever decreases with attachment of the cell and any further changes in the mass of a cell shifts the resonance frequency of the cantilever accordingly. A schematic of the principles of the pico-balance is shown in Figure 4. | |
 |
|
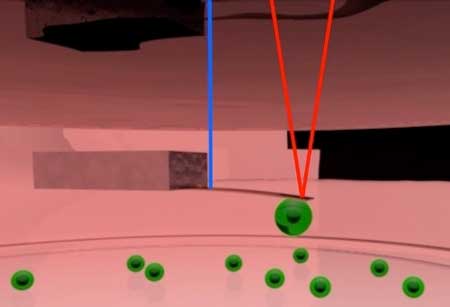
| Figure 4. Schematic of pico-balance setup. The cantilever is oscillated at about 0.5 nm amplitude using an intensity modulated laser at the base of the cantilever (Blue). Another laser focused on the free end of the cantilever (red) is used to monitor the resonance frequency of the cantilever. The mass of the cell attached to the cantilever can be derived from the resonance frequency. | |
| Cell mass development can easily be affected by sub-optimal environmental conditions. To prevent this, a controlled environmental system was developed to maintain the viability of the cells and monitor the cell mass for multiple days. The system is equipped with a special sample holder controlling humidity, temperature, and CO2, acting as a miniature cell culture incubator. A schematic of this setup is shown in Figure 5. | |
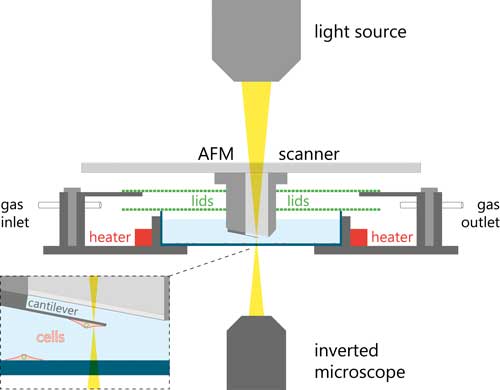 |
|
| Figure 5. Schematic of the pico-balance setup. (click on image to enlarge) | |
| The investigators chose HeLa cells as a model system and attached the cells to collagen I coated cantilevers. | |
| To study effect of a virus infection on the cell mass, a single cantilever-bound HeLa cell was brought in contact with a vaccinia virus (VACV) infected BSC 40 cells seeded on the bottom of the incubator cell. The cantilever-bound cell could thus be selectively infected. | |
| The cells were infected with a recombinant vaccinia virus containing the eGFP-tagged core protein A4 to follow new virus particle production by fluorescence microscopy. The fluorescence intensity of the infected cells increased over time indicating that new virus particles were being formed in the cells. Both control and infected HeLa cells showed that the mass of the attached cells fluctuated periodically by a few percent at a timescale in the few seconds range. | |
| However, the mass of the infected cells remained constant over longer periods and did not enter mitosis. Control cells that were not brought in contact with infected cells gradually increased mass and divided on the cantilever (Figure 6). | |
 |
|
| Figure 6. Long-term cultivation and mass monitoring of control (black trace) and virus-infected HeLa cells (gray trace). Cells were infected by bringing the cell attached to the cantilever in contact with an infected cell in the Petri dish. The dips in the black trace show where mitosis occured, during which cells rounded up and adhered only weakly to the cantilever. | |
Conclusion |
|
| AFM is a powerful technique to analyze and manipulate biological samples under physiological conditions. It can be combined with advanced optical techniques, to allow correlation of optical characterization of the sample cells in parallel to the AFM experiments. | |
| In this application we showed two studies of host-virus interactions at the single cell level. In the first example the AFM was used as a manipulation tool using FluidFM technology. In the second example the mass development of virus-infected cells was studied after infection. | |
| Virus research in the past has led to the development of treatments and vaccines. New viruses like the recent SARS-CoV2, which has led to the COVID-19 pandemic, stressing the importance of continued virus research to become a better and faster response to future outbreaks. | |
| References | |
| Force-controlled manipulation of single cells: from AFM to FluidFM. O. GuillaumeGentil, E. Potthoff, D.Ossola, C.M. Franz, T.Zambelli and J.A. Vorholt. Trends in Biotech. 2014. 32(7), 381-388. | |
| Cooperative Vaccinia Infection Demonstrated at the Single-Cell Level Using FluidFM. P. Stiefel, F.I. Schmidt, P.Dörig, P.Behr, T. Zambelli, J. A. Vorholt, and J. Mercer. Nano Letters 2012, 12(8), 4219-4227. | |
| Inertial picobalance reveals fast mass fluctuations in mammalian cells. D. Martínez-Martín, G. Fläschner, B. Gaub, S. Martin, R. Newton, C. Beerli, J. Mercer, C. Gerber & D. J. Müller. Nature. 2017. 550, 500– 505 | |
| Provided by Nanosurf AG | |
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
