| Jun 23, 2021 | |
Sustainable polymer nanocoatings: An innovative concept |
|
| (Nanowerk Spotlight) The modification of surface properties, such as metals, medical devices, and glass mirrors, represents a vital opportunity to inhibit the buildup of proteins or contaminants (i.e., fouling) for biomedical materials/devices, food packaging, many membrane filtration applications. Surface coatings with sustainable anti-fouling, bactericidal or the killing of bacterial membrane cells, and self-cleaning surface properties are particularly desirable and are receiving great attentions. | |
| Clinical results have depicted the development of catheter-related infections, such as thrombosis, caused by numerous types of bacteria which adhere onto a fibrin and a vascular catheter, form colonies, and produce coagulating enzymes, leading to mural thrombosis1. | |
| Additionally, the adsorption of blood proteins onto biomedical devices (such as sensors or implants) may reduce effectiveness or even prompt unwanted biological responses2. | |
| Although an anticoagulant drug treatment offers an opportunity to utilize these internal devices, the drug therapy often consists of undesirable side effects including hemorrhage, neutropenia, and thrombocytopenia3. | |
| Polymer-based coatings involving fabrications of nanostructured surfaces over conventional chemical-based antimicrobial agents have become major research efforts designed to greatly prevent protein/bacteria adsorption as well as to promote self-cleaning surface abilities for numerous industry applications. | |
| A better understanding of the molecular-level mechanism by which polymer coatings lead to anti-fouling surface properties is vital. It has been believed that “interfacial water molecules”, which strongly interact with a hydrophilic polymer surface, act as a “barrier” against protein adsorption. Hence, as Berg4 and Vogler et al.5 proposed, a water contact angle can be used as a gauge to connect the process of protein adsorption. | |
| Generally, hydrophilic surfaces are those in which the water contact angle θ<90°, while hydrophobic surfaces are characterized by water contact angles of θ>90° 6. | |
| Quantitatively, Berg’s limit defines a critical contact angle of θ≅65° in which hydrophilic surfaces with any angle θ less than the critical value are able to resist protein absorption as the protein cannot displace the water from the surface3,4. | |
| In essence, the hydration forces found in the water layer bound to a surface repels protein adhesion5, and if the hydration forces are enhanced through greater attraction from a modified surface such as involving carefully structured polymers, the repulsion force can also be increased. | |
| Understanding this relationship prompts the development of sustainable surface modifications especially involving optimally designed polymers which must resist protein absorption both chemically and mechanically and represents a sustainable option over a long duration of time. | |
| The design of protein-resistant self-assembled monolayers (SAMs) follows a set of rules termed the Whitesides rules, which describe optimal functional groups aimed towards discouraging protein adsorption7,8. | |
| As simplified by Haag et al.3, the Whitesides rules proposed that 1) the presence of polar functional groups or hydrophilicity, 2) the presence of hydrogen bond acceptor groups, 3) the absence of hydrogen bond donor groups, and 4) the absence of net charge within the molecular structure of polymer prevents protein adsorption7,8. | |
| Thereafter, various optimally designed polymers, such as polyglycerol (PG)10 and zwitterionic polymers9, exhibited excellent resistance to protein adsorption. Importantly, although the molecular structure of PG contains many hydroxyl groups acting as hydrogen bond donors seemingly violating the third Whitesides rule, the remarkable hydrophilicity of a surface coated with PG makes up for an exception3. | |
| Particularly, zwitterionic molecules are chemically composed of an equal number of charged functional groups, which promotes ion-ion or ion-dipole bonding with water rather than simply weaker hydrogen bonding found in other polymers like PG3. As aforementioned, this greater attraction between the polymers atop the modified surface and water results in greater repulsion forces. | |
| Recently, Endoh and Koga11 reported that the nanometer-scale structural barrier associated with polymer conformations governs protein resistance of polymer surfaces instead of interfacial chemical interactions. | |
| The anti-fouling polymer coating they designed is composed of non-charged, hydrophilic or hydrophobic homopolymer chains physically adsorbed onto a solid, resulting in a few nanometer-thick polymer layer (“polymer nanolayer”). | |
| To understand the mechanism behind the undiscovered protein resistant property of the adsorbed polymer chains, they investigated the chain conformations of this polymer nanolayer in water using sum frequency generation spectroscopy (SFG) and explicit solvent coarse-grained molecular dynamics (MD) simulations. | |
 |
|
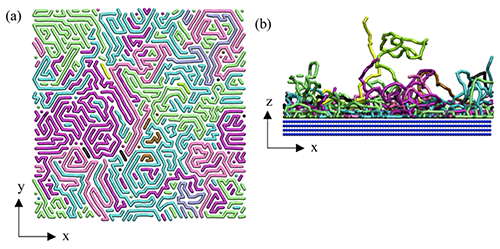
| Figure 1. MD simulations of polymer chains (a) depicting compact nematic order of trains within the plane of the substrate and (b) outer plane brush-like conformations of polymer chain loops and tails. (Reprinted with permission by American Chemical Society from reference 11 below) | |
| The SFG results clarified the non-significant role of the interfacial water in the emergence of an anti-fouling property and the two-dimensional chain architecture commonly shared among the polymers in water. The MD results further allowed us to establish the generality of the self-organized polymer architecture in water: outer high-density “loops” (sequences of free segments connecting successive trains) and “tails” (non-adsorbed chain ends) and inner densely packed “trains” (adsorbed segments) across homopolymer systems with different interactions among a polymer, substrate, and water. | |
| Hence, it is hypothesized that the loops/tails act as high-density polydispersed brushes and the trains behave as a molecular level “rigid-wall”, such that most of the proteins are not allowed to penetrate into the nanolayer. | |
| Thus, their findings not only provide a molecular level understanding of the mechanism behind protein adsorption but also facilitates a universal structure-based design of anti-fouling surfaces, which is currently lacking, using common synthetic polymers. In addition, it is expected that the initial adhesion of bacteria, whose mechanism is generally protein-mediated, can be prevented by these antifouling materials. | |
| Endoh and Koga17 further extended their studies on ultrathin homopolymer films (up to 60 nm thick) composed of polystyrene (PS), poly(2-vinyl pyridine) (P2VP), poly(methyl methacrylate) (PMMA), and polybutadiene (PB). Protein adsorption test against two model plasma proteins were subject: bovine serum albumin (BSA) and fibrinogen. The two proteins have significant differences in their size, shape, and internal stability. | |
| Their experimental results reveal that protein adsorption within all the ultrathin films is almost prohibited when the film thickness (h) is less than a critical thickness hc≅ 20 nm), while at h>hc, the amount of protein adsorption exhibits very strong thickness dependence (αh2). | |
| Molecular dynamics simulations identified a correlation between protein adsorption and highly packed conformations of polymer chains, which either adsorb on the substrate or do not adsorb but are in contact with the adsorbed polymer chains, resulting in a protein repellent “dense layer” at h<hc_c. In addition, the effect of the dense layer propagates into the film interior up to at least 60 nm, resulting in an “interphase” that shows the quadratic adsorption behavior. | |
| The results thus far point to a new principle in protein adsorption within a viscoelastic medium under nanoconfinement. | |
| The utilization of highly dense polymer brush coatings using specialized PG polymers and benzophenone has particularly shown to be sustainable over a long duration of time, applicable to the most non-polar surfaces such as PS, poly(dimethylsiloxane) (PDMS) and poly(tetrafluoroethylene) (PTFE), as well as result in up to 97% repulsion of proteins found in complex solutions of fetal bovine serum and human blood plasma2. | |
| Additionally, although the coated surfaces were characteristically bioinert, the molecular modifications of the specialized PG polymers enabled bio-specific interaction for desired bacteria. Specifically, the addition of α-D Mannose grafted onto the specialized PG polymers allowed for specific adsorption of E. coli, which is a vital development for utilization in filtration, wastewater treatment, and numerous other commercial applications2. | |
| An alternative approach towards preventing biofilm formation is the process of bactericide, or the killing of bacterial cells, commonly resulting from certain nano-topological modifications to surfaces. | |
| One of such modifications follows a biomimetic approach, mimicking the architecture of a cicada wing18, or other nature-inspired surfaces mimic shark-skin/gecko feet19,20. | |
 |
|
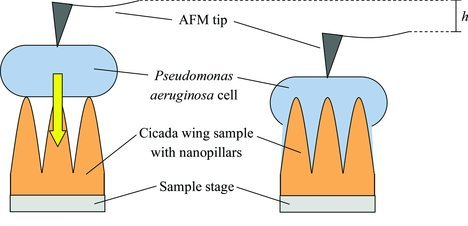
| Figure 2. Illustration of the mechanical sinking process resulting in bactericidal effects involving a P. aeruginosa cell in contact with a cicada wing nanopillar. (Reprinted with permission by Wiley-VCH Verlag from reference 18 below) | |
| Figure 2 illustrates a simplification of the mechanical process by which a cicada-wing nanopillar interacts with P. aeruginosa. Upon initial contact, the bacterial cell membrane is subject to adhesion forces between adjacent nanopillars and is thereafter stretched and eventually deflated, leading to cell death18,21. | |
| Scanning electron microscopy (SEM) imaging revealed that many that high numbers of P. aeruginosa bacterial cells indeed adhered to the surface of cicada wings, however, were killed within a few minutes, resulting in an “antibiofouling” property18. | |
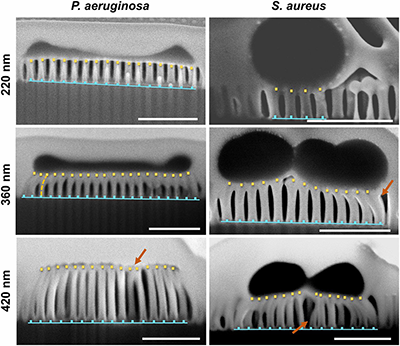
| Enhancement of the bactericidal effects of certain nano-topologies hinges on physical parameters including sizes and heights. Figure 3 represents focused ion-beaming – scanning electron microscopy (FIB-SEM) imaging of plasma etched nanopillars with varying height of 220, 360, and 420 nm in contact with either P. aeruginosa or S. aeruginosa21. | |
 |
|
| Figure 3. FIB-SEM imaging of the substrate with 220, 360, 420 nm long nanopillars exposed to P. aeruginosa or S. aeruginosa, in which arrows indicate positions of Clustering nanopillars. (Reprinted with permission by National Academy of Sciences from reference 21 below) (click on image to enlarge) | |
| Importantly, as compared to the structure of bacteria exposed to flat silicon control surfaces, those exposed to surfaces consisting of the nanopillars were deflated or elongated, providing further direct evidence of the mechanical process depicted in Figure 2 21. | |
| For nanopillar heights of 220, 360, and 420 nm, the average proportion of dead bacterial cells was 53%, 95%, and 89%, respectively. The enhanced bactericidal effect of 360 nm as compared to 220 nm was attributed to greater stress-induced deflection forces on bacterial membranes which generates greater elongation or stretching. | |
| Clearly, the bactericidal effects decreased for the nanopillar length of 420 nm while depicting greater cluster formations in FIB-SEM imaging in Figure 3. Mechanically, longer nanopillars are more flexible and prone to collapse under stress by the weight of bacterial cells. As compared to nanopillars, the clusters have greater surface area and are not as effective at inducing deflection forces21. | |
| Anti-fouling and self-cleaning properties extend far greater than biomedical applications. The key to creating self-cleaning surfaces is derived from nature, in particular, the Lotus leaf. The Lotus leaf is non-wettable, with a characteristic self-cleaning mechanism in which the droplets running of a waxy surface carries dry contaminants6. | |
| Self-cleaning surfaces and water repellency are especially favorable in glass manufacturing, oil-water separation, and corrosion protection22. The combination of polymethylsiloxane (PDMS) and silica (SiO2) in a simple spray-coating technique have shown to form a rough, superhydrophobic surface (the water contact angle of 156.4°) which withstood both sand abrasion and water impact22. | |
| Although stable, primary predicament surrounding commercial applications is the decrease of light transmittance as a result of surface coating, which is especially detrimental for applications towards self-cleaning windows6. Few studies evaluate or comment on the aging effects on these newly developed coatings. As such, sustainable anti-fouling surfaces must be analyzed over a prolonged period of contamination in order to truly gauge the effectiveness of certain modified surfaces. | |
| Provided by Dr. Raj Shah, Dr. Tad Koga and Mr. Blerim Gashi as a Nanowerk exclusive. | |
About the Authors |
|
| Dr. Raj Shah is currently the Director at Koehler Instrument Company in New York, and is a veteran of the petrochemical industry for the last 25 plus years. He has over 400 publications and is an elected Fellow by his peers at IChemE, CMI, STLE, AIC, NLGI, INSTMC, The Energy Institute and The Royal Society of Chemistry. He can be reached at [email protected] | |
| Dr. Tad Koga is a full professor, with a joint appointment in Dept. of Material Science and Chemical Engineering, and Chemistry departments at The State University of New York, Stony Brook, and has over two and half decades of experience in the field of sustainable polymers and nanoparticles. His research interest is the development of “green” energy, manufacturing and processing: (i) green nanofabrication of polymer thin films using supercritical carbon dioxide, (ii) chemical recycling of waste plastic using supercritical water, and (iii) methane hydrate as a future energy resource | |
| Mr. Blerim Gashi is a student of Chemical engineering at SUNY, Stony Brook University, where Dr. Shah is an adjunct professor and the chair of the external advisory Committee in the Dept. of Material Science and Chemical Engineering. | |
References |
|
| [1] Raad, I. Issam & Luna, Mario & Khalil, M. Sayed-Ahmed & Costerton, W. John & Lam, Chau & Bodey, P. Gerald. (1994). The Relationship Between the Thrombotic and Infectious Complications of Central Venous Catheters. JAMA: The Journal of the American Medical Association, 271(13), 1014. doi:10.1001/jama.1994.03510370066034 | |
| [2] Yu, Leixiao & Hou, Yong & Cheng, Chong & Schlaich, Christoph & Noeske, P.-L & Wei, Qiang & Haag, Rainer. (2017). High-Antifouling Polymer Brush Coatings on Nonpolar Surfaces via Adsorption-Cross-Linking Strategy. ACS Applied Materials & Interfaces. 9. 10.1021/acsami.7b13515. | |
| [3] Wei, Qiang & Becherer, Tobias & Angioletti-Uberti, Stefano & Dzubiella, Joachim & Wischke, Christian & Neffe, Axel & Lendlein, Andreas & Ballauff, Matthias & Haag, Rainer. (2014). Cover Picture: Protein Interactions with Polymer Coatings and Biomaterials (Angew. Chem. Int. Ed. 31/2014). Angewandte Chemie International Edition. 53. 7959-7959. 10.1002/anie.201406350. | |
| [4] Berg, Johan & Eriksson, L. & Claesson, Per & Borve, Kari. (1994). Three-Component Langmuir-Blodgett Films with a Controllable Degree of Polarity. Langmuir. 10. 10.1021/la00016a041. | |
| [5] Vogler EA. Structure and reactivity of water at biomaterial surfaces. Adv Colloid Interface Sci. 1998 Feb;74:69-117. doi: 10.1016/s0001-8686(97)00040-7. PMID: 9561719. | |
| [6] Blossey, Ralf. (2003). Self-Cleaning Surfaces—Virtual Realities. Nature Materials. 2. 301-6. 10.1038/nmat856. | |
| [7] Ostuni, Emanuele & Chapman, Robert & Holmlin, R. & Takayama, Shuichi & Whitesides, George. (2001). A Survey of Structure−Property Relationships of Surfaces that Resist the Adsorption of Protein. Langmuir. 17. 10.1021/la010384m. | |
| [8] Chapman, Robert & Ostuni, Emanuele & Takayama, Shuichi & Holmlin, R. & Yan, Lin & Whitesides, George. (2000). Surveying for Surfaces that Resist the Adsorption of Proteins. Journal of The American Chemical Society - JACS. 122. 10.1021/ja000774f. | |
| [9] Jiang, S., & Cao, Z. (2010). Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Advanced Materials, 22(9), 920–932. https://doi.org/10.1002/adma.200901407 | |
| [10] Wyszogrodzka, M., & Haag, R. (2009). Synthesis and characterization of glycerol dendrons, self-assembled monolayers on gold: a detailed study of their protein | |
| [11] Endoh, Maya & Morimitsu, Yuma & Salatto, Daniel & Huang, Zhixing & Sen, Mani & Li, Weiyi & Meng, Yizhi & Thanassi, David & Carrillo, Jan-Michael & Sumpter, Bobby & Kawaguchi, Daisuke & Tanaka, Keiji & Koga, Tadanori. (2019). Protein Resistance Driven by Polymer Nanoarchitecture. ACS Macro Letters. 8. 1153-1159. 10.1021/acsmacrolett.9b00518. | |
| [12] Sen, Mani & Jiang, Naisheng & Endoh, Maya & Koga, Tadanori & Ribbe, Alexander & Rahman, Atikur & Kawaguchi, Daisuke & Tanaka, Keiji & Smilgies, D.-M. (2018). Locally Favored Two-Dimensional Structures of Block Copolymer Melts on Nonneutral Surfaces. Macromolecules. 51. 10.1021/acs.macromol.7b02506. | |
| [13] Gin, Peter & Jiang, Naisheng & Liang, Chen & Taniguchi, Takashi & Akgun, Bulent & Satija, Sushil & Endoh, Maya & Koga, Tadanori. (2012). Revealed Architectures of Adsorbed Polymer Chains at Solid-Polymer Melt Interfaces. Physical Review Letters. 109. 265501. 10.1103/PhysRevLett.109.265501. | |
| [14] Jiang, Naisheng & Shang, Jun & Di, Xiaoyu & Endoh, Maya & Koga, Tadanori. (2014). Formation Mechanism of High-Density, Flattened Polymer Nanolayers Adsorbed on Planar Solids. Macromolecules. 47. 2682-2689. 10.1021/ma5003485. | |
| [15] Sen, Mani & Jiang, Naisheng & Cheung, Justin & Koga, Maya & Koga, Tadanori & Kawaguchi, Daisuke & Tanaka, Keiji. (2016). Flattening Process of Polymer Chains Irreversibly Adsorbed on a Solid. ACS Macro Letters. 5. 504-508. 10.1021/acsmacrolett.6b00169. | |
| [16] Aubouy, M., Fredrickson, G. H., Pincus, P., & Raphaeel, E. (1995). End-Tethered Chains in Polymeric Matrixes. Macromolecules, 28(8), 2979–2981. doi:10.1021/ma00112a051 | |
| [17] Salatto, Daniel & Koga, Yuto & Bajaj, Yashasvi & Huang, Zhixing & Yavitt, Benjamin & Meng, Yizhi & Carrillo, Jan-Michael & Sumpter, Bobby & Nykypanchuk, Dmytro & Taniguchi, Takashi & Endoh, Maya & Koga, Tadanori. (2020). Generalized Protein-Repellent Properties of Ultrathin Homopolymer Films. Macromolecules. 53. 10.1021/acs.macromol.0c01010. | |
| [18] Ivanova, Elena & Hasan, Jafar & Webb, Hayden & Truong, Vi Khanh & Watson, Gregory & Watson, Jolanta & Baulin, Vladimir & Pogodin, Sergey & Wang, James & Tobin, Mark & Löbbe, Christian & Crawford, Russell. (2012). Natural Bactericidal Surfaces: Mechanical Rupture of Pseudomonas aeruginosa Cells by Cicada Wings. Small. 8. 2489-94. 10.1002/smll.201200528. | |
| [19] Jung, Y. C., & Bhushan, B. (2009). Wetting behavior of water and oil droplets in three-phase interfaces for hydrophobicity/philicity and oleophobicity/philicity. Langmuir: the ACS journal of surfaces and colloids, 25(24), 14165–14173. https://doi.org/10.1021/la901906h resistance. Biomacromolecules, 10(5), 1043–1054. https://doi.org/10.1021/bm801093t | |
| [20] Lee, H., Lee, B. P., & Messersmith, P. B. (2007). A reversible wet/dry adhesive inspired by mussels and geckos. Nature, 448(7151), 338–341. https://doi.org/10.1038/nature05968 | |
| [21] Ivanova, Elena & Linklater, Denver & Werner, Marco & Baulin, Vladimir & Xu, Xiumei & Vrancken, Nandi & Rubanov, S. & Hanssen, Eric & Wandiyanto, Jason & Truong, Vi Khanh & Elbourne, Aaron & Maclaughlin, Shane & Juodkazis, Saulius & Crawford, Russell. (2020). The multi-faceted mechano-bactericidal mechanism of nanostructured surfaces. PNAS. 10.1073/pnas.1916680117. | |
| [22] Gong, X., & He, S. (2020). Highly Durable Superhydrophobic Polydimethylsiloxane/Silica Nanocomposite Surfaces with Good Self-Cleaning Ability. ACS Omega, 5(8), 4100–4108. https://doi.org/10.1021/acsomega.9b03775 | |
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
