Nanochemistry: The Bridge Between Nanoscience and Chemical Reactions
Definition of Nanochemistry
Nanochemistry is a branch of nanoscience that focuses on the study and manipulation of chemical reactions at the nanoscale, typically in the range of 1-100 nanometers. It involves the synthesis, characterization, and application of nanomaterials with unique chemical properties that arise from their small size and high surface-to-volume ratio.
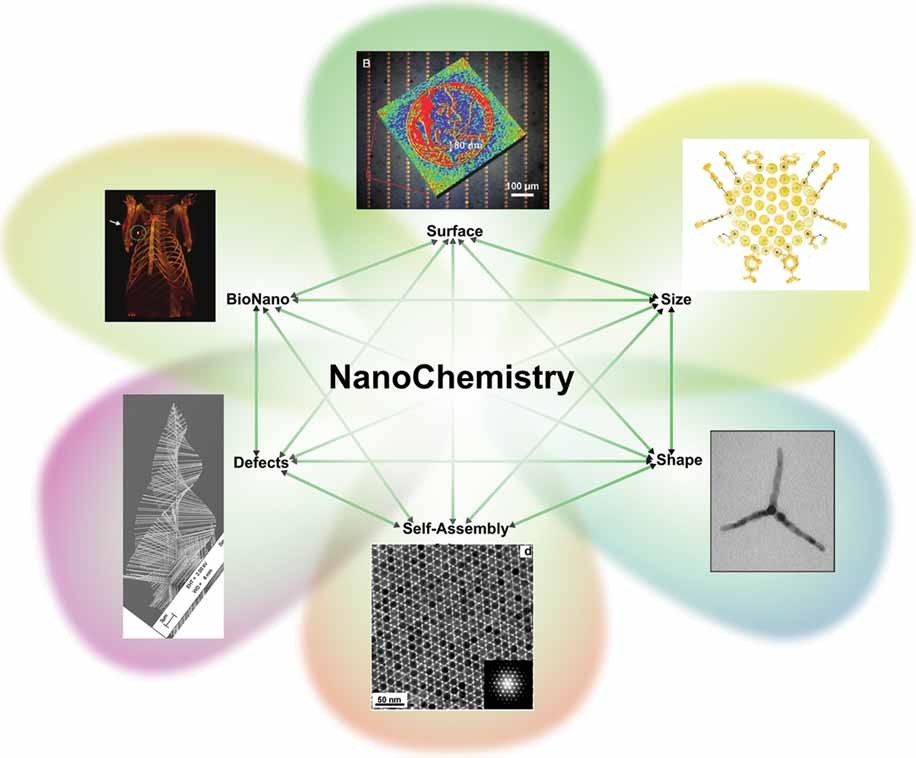
Key Concepts in Nanochemistry
Nanochemistry revolves around several key concepts that distinguish it from traditional chemistry:
Size-Dependent Properties
As materials are reduced to the nanoscale, their chemical and physical properties can change dramatically compared to their bulk counterparts. This is due to quantum confinement effects and the increased influence of surface atoms. By precisely controlling the size of nanomaterials, nanochemists can fine-tune their electronic, optical, and catalytic properties.
Surface Chemistry
The high surface-to-volume ratio of nanomaterials makes surface chemistry a crucial aspect of nanochemistry. The reactivity, adsorption, and catalytic properties of nanomaterials are largely determined by the chemical composition and structure of their surfaces. Nanochemists can engineer the surface of nanomaterials through functionalization, passivation, or coating to tailor their chemical behavior.
Self-Assembly and Bottom-Up Synthesis
Nanochemistry often employs bottom-up synthesis approaches, where nanomaterials are built from smaller building blocks through self-assembly processes. By carefully designing the chemical interactions between the building blocks, nanochemists can create complex nanostructures with precise control over their size, shape, and composition. Examples include the synthesis of quantum dots, nanowires, and nanoparticle superlattices.
Applications of Nanochemistry
Nanochemistry has a wide range of applications across various fields:
Catalysis
Nanomaterials with high surface areas and unique electronic properties can serve as highly efficient catalysts for chemical reactions. Nanochemists design and synthesize nanocatalysts with improved activity, selectivity, and stability compared to conventional catalysts. Applications include industrial processes, energy conversion, and environmental remediation.
Sensing and Detection
Nanomaterials with tailored chemical and optical properties can be used as sensitive and selective sensors for various analytes, such as pollutants, biomolecules, or gases. Nanochemists develop nanoscale sensors based on mechanisms like surface-enhanced Raman scattering, fluorescence quenching, or electrochemical detection. These nanosensors find applications in environmental monitoring, medical diagnostics, and food safety.
Drug Delivery and Nanomedicine
Nanochemistry plays a crucial role in developing targeted and controlled drug delivery systems. Nanomaterials like polymeric nanoparticles, liposomes, and mesoporous silica can encapsulate drugs and release them at specific sites in the body. Nanochemists design these nanocarriers with stimuli-responsive properties, biocompatibility, and enhanced permeability and retention effect. Nanomedicine applications include cancer therapy, gene delivery, and tissue engineering.
Energy Storage and Conversion
Nanochemistry contributes to the development of advanced materials for energy storage and conversion devices. Nanomaterials with high surface areas, fast ion transport, and improved stability are used in lithium-ion batteries, supercapacitors, and fuel cells. Nanochemists also explore nanomaterials for photovoltaics, thermoelectrics, and hydrogen storage to enable clean and sustainable energy solutions.
Challenges and Future Perspectives
Despite the remarkable progress in nanochemistry, several challenges remain. One major challenge is the scalable and reproducible synthesis of nanomaterials with precise control over their properties. Achieving uniform size distribution, purity, and stability of nanomaterials is essential for their reliable performance in various applications.
Another challenge is understanding and predicting the behavior of nanomaterials in complex environments, such as biological systems or under extreme conditions. Nanochemists need to develop advanced characterization techniques and computational models to probe the structure-property relationships of nanomaterials and guide their rational design.
Future research in nanochemistry will focus on developing multifunctional and adaptive nanomaterials that can respond to external stimuli or self-repair. The integration of nanochemistry with other disciplines, such as biology, electronics, and robotics, will open up new possibilities for smart and autonomous nanoscale systems. Additionally, the environmental and health impacts of nanomaterials need to be thoroughly assessed to ensure their safe and responsible use.
Further Reading
Chemical Society Reviews, Nanochemistry and nanomaterials for photovoltaics
Materials, Surface Chemistry in Nanoscale Materials
