| Posted: Jul 04, 2014 |
The first single-component molecular superconductor emerges under pressure
|
|
(Nanowerk News) Three decades ago, researchers discovered that certain organic molecules become superconducting at low temperatures. This finding sparked numerous investigations into the properties of these lightweight, low-cost and easy-to-modify materials. Despite much recent progress, chemists remain puzzled by one aspect of these compounds: all known molecular superconductors need the cooperative action of two or more different molecular species to move electrons without resistance.
|
|
HengBo Cui and Reizo Kato from the RIKEN Condensed Molecular Materials Laboratory in collaboration with Hayao Kobayashi and Akiko Kobayashi from Nihon University have now realized a crucial goal in the search for metal-like organic molecules by uncovering the first molecular superconductor containing only one component ("A Single-Component Molecular Superconductor").
|
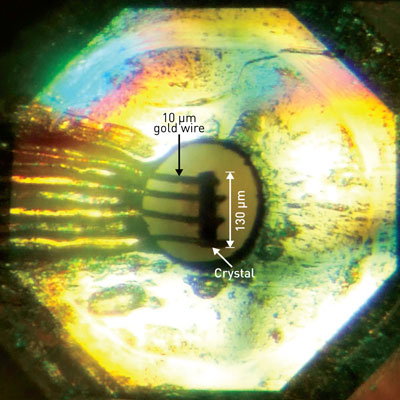 |
| Figure 1: The diamond anvil cell used to induce superconductivity in a single-component nickel–organic molecule. (© American Chemical Society)
|
|
Superconducting organic crystals are designed around the principle of charge-transfer complexes, where strong interactions between distinct ‘donor’ and ‘acceptor’ components move electrons through normally insulating carbon bonds. By squeezing the charge-transfer structures together using diamond anvil cells—tools that allow crystals to be compressed at pressures of up to millions of atmospheres—resistance-free electrical transport can occur at temperatures near absolute zero.
|
|
The electron donors and acceptors in molecular superconductors are normally individual ionic compounds. However, Kobayashi’s team has recently spearheaded investigations into metal–dithiolate complexes that contain a complete charge-transfer system in a single molecule. These crystals, in which a central gold or nickel acceptor atom is flanked on two sides by extended aromatic donor rings infused with sulfur atoms, have a high intrinsic conductivity and exhibit metallic behavior at low temperatures.
|
|
The researchers partnered with Masaaki Sasa from Fujitsu to explore numerous metal–dithiolate synthetic derivatives. They eventually found a promising compound, nickel bis(trifluoromethyl)tetrathiafulvalenedithiolate (Ni(hfdt)2). This molecule has bulky fluorinated end-groups on its dithiolate rings that trigger two-dimensional layer stacking in the crystal state—a highly favorable arrangement for metal-like conductivity.
|
|
After carefully manipulating the tiny, submillimeter-sized Ni(hfdt)2 crystals into their diamond anvil cell device (Fig. 1), the team measured how its electrical behavior changed with pressure and temperature. At a pressure of about 8.1 gigapascals, they found that the resistivity suddenly plunged to zero at a temperature of 5.5 kelvin—clear evidence that they had discovered a single-component molecular superconductor. High-level theoretical calculations confirmed these experimental findings by revealing the critical point at which pressure converts Ni(hfdt)2 from an insulator to a superconductor.
|
|
“This simple, single-component compound not only has the potential to bring about breakthroughs in organic solid-state devices, but will also help in the design of new superconducting systems,” says Cui.
|

