| Posted: Feb 21, 2011 | |
Nanotechnology-based solutions for oil spills |
|
| (Nanowerk Spotlight) The recent oil spill in the Gulf of Mexico is widely acknowledged to be among the worst ocean oil spills in world history. Inevitably, the spill has once again raised serious concerns worldwide about the likely environmental impact of such catastrophic oil spills caused by oil tanker accidents at sea or mishaps during loading and unloading of oil from tankers at seaports. Similar concerns are also associated with discharge of oil in areas around oil wells and oil storage facilities. Such oil spills can cause havoc to marine ecology (sea birds, mammals, algae, coral, seagrass etc.), beside the health hazards to the human population located in nearby coastal zones. Moreover, the economic loss suffered by oil companies resulting from oil-spillage is enormous. | |
|
Numerous solutions have been proposed for dealing with the problem of oil spills. These include
|
|
| Conventional techniques are not adequate to solve the problem of massive oil spills. In recent years, nanotechnology has emerged as a potential source of novel solutions to many of the world's outstanding problems. Although the application of nanotechnology for oil spill cleanup is still in its nascent stage, it offers great promise for the future. In the last couple of years, there has been particularly growing interest worldwide in exploring ways of finding suitable solutions to clean up oil spills through use of nanomaterials. | |
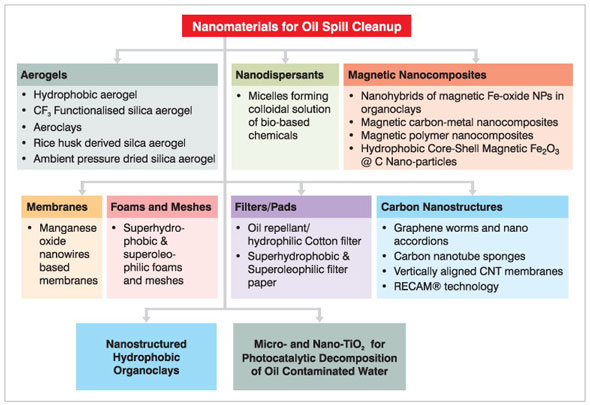 |
|
| Figure 1: Various approaches for oil spill cleanup using nanomaterials/nanotechnologies. | |
| Importance of wettability for water-oil separation | |
| Solid surfaces with unique wettability characteristics combining superhydrophobicity (water contact angle (CA) greater than 1500) along with superoleophilicity (oil CA smaller than 5°), have attracted great deal of interest in the field of marine coatings, microfluidic devices, self-cleaning surfaces etc 1-3. Such surfaces have especially significant potential for the possible separation of oil and water. Superhydrophobicity is an outcome of a combination of intrinsic hydrophobic properties of the material (chemical composition) that constitutes the surface, as well as the microscale and nanoscale roughness of that surface. | |
| Figure 1 above schematically shows the various approaches employed for oil spill cleanup using nanomaterials. The present article specifically presents an overview of these approaches. | |
| Nanomaterials and Technologies | |
| Aerogels | |
|
– Hydrophobic silica aerogels (e.g.,Nanogel@aerogel) – Aeroclays – Maerogel (Rice Husk Derived Aerogel) |
|
| Nano Dispersants | |
| One of the technologies used for oil spill response involves the use of chemical dispersants which contain surfactant molecules (surface-active agents) that migrate to the oil/water interface and reduce interfacial tension between oil and water. With the aid of wave energy, tiny oil droplets break away from the oil slick. These small droplets get dispersed into the water column and remain in suspension and, thereby, become a good source of food for the naturally occurring bacteria. The dispersants catalyse the biodegradation process leading to the removal of spilled oil. | |
| Hydrophobic Organoclays | |
| Natural clays like bentonites contain metallic cations, which impart hydrophilic character to the clay. Therefore, they are not suitable sorbents for the removal of organic compounds. However, hydrophobicity can be induced in these clays by modifying them with quaternary amines (a type of surfactant containing nitrogen ion) 4,5. The presence of these amines render the clay hydrophobic in nature. These organophilic clays (or organoclays) are very efficient in selectively adsorbing the organic contaminants or oil from water. | |
| Magnetic Materials | |
|
– Magnetic Nanocomposites – Hydrophobic Core-Shell Magnetic Fe2O3@C Nanoparticles – Magnetic Polymer Nanocomposites – Magnetic Carbon Composites – Organo-clays with Magnetic Fe3O4 Nanoparticles – Magnetic Liquid Foams; EcoMag® and CleanMag® |
|
| Nanowire Membranes | |
| MIT researchers have developed absorbent, superhydrophobic nanowire membranes for the selective absorption of oil from an oil-water mixture 6. Using self assembly method, they have constructed free-standing membranes comprising inorganic nanowires capable of absorbing oil up to 20 times their weight. | |
| By employing the above mentioned nano-wire mesh, MIT's SENSEableCity Laboratory has recently created an autonomous oil-absorbing robot called Sea-swarm 7. This prototype robot uses a conveyor belt covered with the oil absorbing nano-wire mesh. When Sea-swarm moves along the surface of water, the conveyor belt along with the nano mesh rotates, and selectively absorbs the water to do the cleaning job. These autonomous vehicles use very little energy (as low as about 100 watts), run for weeks and have the capacity to clean up several gallons of oil per hour. | |
| Carbon Nanostructures | |
|
– Exfoliated Graphite – Carbon Nanotube Sponge – Vertically Aligned CNTs (VACNTs) for Combating Oil Spills – Graphene Worms and Nano Accordions Derived from thermally exfoliated graphite oxides TEGO – RECAM® Technology is a novel reactive nanostructured carbon material. – High Reactivity Carbon Mixture (HRCM) Sorbent. |
|
| Micro-and Nano-TiO2 for Oil Spill Remediation | |
| Oil spills generally result in contamination of seawater due to the dissolution of water-soluble crude oil fractions. This contaminated water, rich in dissolved hydrocarbons, is highly toxic in nature and can cause irreparable damage to the coastal ecosystem. Photocatalytic decomposition of the oil-contaminated water using nanoscale or microscale TiO2 particles can provide an effective solution to this problem 8-10. | |
| Cotton Absorbent Pads and Filter Papers | |
| Inexpensive, raw cotton waste is an amazing oil absorbing material that is also biodegradable in nature. It can soak up the oil up to 40 times its weight. Professor S. Ramkumar of The Institute of Environmental and Human Health (TIEHH), Texas Tech University is developing value-added cotton absorbent pads using non-woven materials and nanotechnology 11. Recently, he chemically treated the raw cotton which enhances its oil absorbing capabilities to soak the oil up to 70 times its weight. | |
| DAG-PEG Lipids for Remediation of Oil Contamination | |
| Brian Charles Keller discovered that certain diacylglycerol-polyethyleneglycol (DAG-PEG) lipids such as PEG-12 GDO (glycerol dioleate) and PEG-12 GDM (glycerol dimyristate) can be used to remediate the oil spills 12. He found that, when DAG-PEG lipids are added to the surface of an oil spill in water, the lipids entrap both water and oil from the immediate surroundings and disperse the contents and vesicles into a suspension. Once the oil is entrapped into vesicles, it remains inside the bi-layers of the vesicle and in suspension indefinitely, since the formed vesicles are thermodynamically stable until disrupted by high energy mechanical shear, by enzyme activity or by heat. | |
| Factors in Implementation of Nanotechnology Based Solutions | |
| For the successful implementation in a real world scenario, a number of factors should be carefully considered. The critical issues that need to be examined are given in the following schematic diagram (Fig. 2). | |
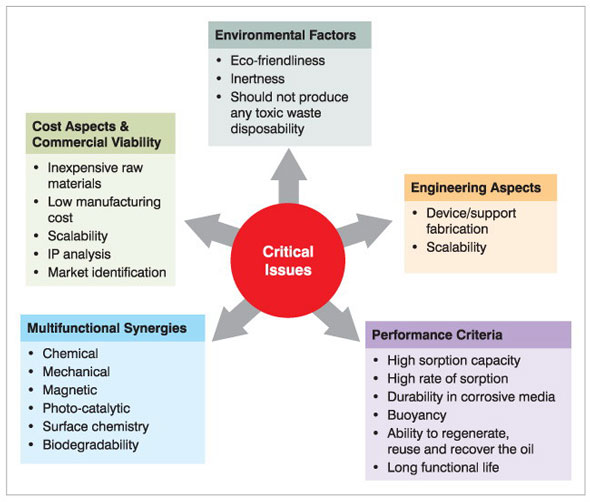 |
|
| Figure 2: Schematic showing the key issues involved in successful implementation of nanotechnology for oil spill remediation. | |
| Performance Criteria | |
| A variety of nano-based products such as membranes, filters, sponges, chemical dispersants etc. play an important role in oil spill remediation. The performance of nanoporous materials for the above application is mainly determined by their sorption capacity, selectivity for organic solvents and oil, rate of sorption, tailored surface chemistry etc. As shown in the bar chart in Fig. 3 below, the nanoporous sorbents, namely thermally exfoliated graphite oxide (TEGO), CF3 functionalized aerogels and carbon nanotube sponges have outstanding oil absorption capacities. Recam® is another nanosorbent having excellent capacity to absorb the oil (90g of oil per 1g of Recam®). One of the most exciting developments in recent months is the discovery of the lightest ever, free-standing multiwall carbon nanotube aerogel3 having a density of 4 mg/cm3 (compared to 5.8-25.5 mg/cm3 for CNT sponge) and a surface area of 580 m3/g (surface area for pristine MWCNT is 241m2/g). Therefore, the ultra light CNT-based aerogels are expected to have much greater potential as a sorbent for oil spill cleanup than CNT sponge. | |
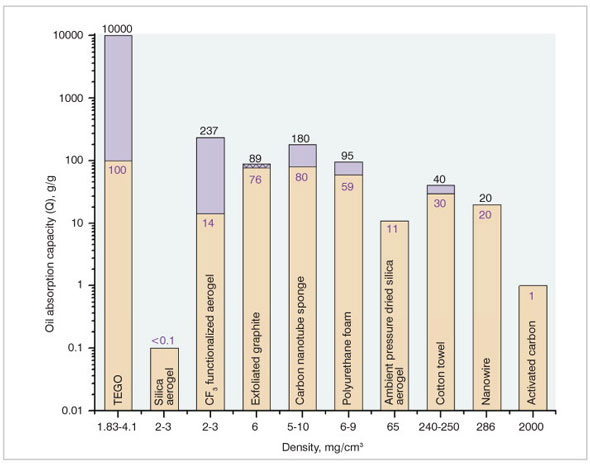 |
|
| Figure 3: Oil absorption capacity of various candidate nanomaterials (different types of oils studied with each material: activated carbon ? diesel oil, CNT sponge ? various organic solvents and oils, PU form ? paraffinic and napthenic oils, CF3 functionalised aerogel ? crude oil, silica aerogel ? crude oil, EG ? different grades of heavy oil, NW ? various types of oils, ambient pressure dried silica aerogel ? diesel oil, TEGO ? not known, cotton towel - not known). | |
| Another important requirement for the sorbent/membrane/filter nanomaterial is its selectivity for a broad range of organic solvents and oils to effectively separate oil and water and thus clean up the oil spills. Conventional sorbents based on polypropylene, silicon-coated glass fibers, raw cotton etc. have a tendency to absorb both water and organic solvents, whereas nanomaterials such as CNT sponges, nanowire membranes, Recam®, hydrophobic core-shell magnetic Fe2O3 @ C nano-particles etc. selectively absorb oil from water-oil mixtures because of their unique combination of superhydrophobic and superoleophilic properties. | |
| An additional feature of nanomaterials like CNT sponges and aerogels, Recam®, Gigasorb etc. is that they are robust, highly flexible and capable of withstanding a number of large strain compressive cycles without affecting their properties. These attributes are highly beneficial for recovery of oil as well as regeneration and reuse of the material. | |
| Multifunctional Synergies | |
| Nanomaterials combined with different functional characteristics such as chemical (hydrophobicity, hydrophilicity and oleophilicity), magnetic, mechanical, photocatalytic properties due to their synergistic effect, have enhanced capability for more efficient removal of oil spills from water surface. One such example is a magnetic polymer composite 14, wherein superparamagnetic nanoparticles of maghemite are incorporated within the matrix of an alkyd resin polymer. In this composite, the oil removal capability is the sum of two distinct effects arising from the magnetic force and the chemical affinity between the resin and the oil. Recam® is a commercial product based on nanostructured carbon containing graphene cell and CNTs. SA Envitech s.r.l. company has ingeniously coupled Recam® (containing graphene) with TiO2 nanoparticles (anatase) and developed a new and innovative product 15,16. This unique combination creates synergy between anatase and graphene and dramatically enhances the efficiency of hydrocarbon/oil removal from contaminated water through photocatalytic degradation process. The coupling of TiO2 with graphene enables easy transfer of electrons, which are released following activation by means of illumination, into the graphene. Furthermore, possibility of electron-hole recombination is also significantly reduced. Silica aerogels, too, have significant potential as a candidate material for selective adsorption of oil. However, since they are fragile and prone to cracking during the ambient pressure drying process, there have been attempts to reinforce it with fibers or CNTs. Organozorb® is another novel sorbent containing microbes, developed by Blue Gold, USA, which combines both nano- and bio- technology, and thereby, greatly enhances bioremediation process. | |
| Environmental Factors | |
| Oil spill remediation using engineered nanomaterials is a more effective option than conventional techniques as it leads to improved performance and response. The superior performance of nanomaterials can be attributed to their increased surface area and, in turn, higher reactivity as well as the possibility of in situ treatment. However, at the same time, materials at the nanoscale may pose new toxicological risks due to their greater biological activity and may have negative impact on human health when these nanoparticles are inhaled, absorbed through skin, or ingested. There is also some scientific evidence, showing that the nanoparticles can travel through the food chain from smaller to larger organisms. In addition, they could damage important microbes in the environment. | |
| Unfortunately, in recent times, there has been a kind of hysteria about the harmful effects of nanotechnology being propagated by certain sections of the media, NGOs and the general public due to misinformation and/or lack of understanding 15. In view of these environmental concerns, there is a clear need to carry out detailed studies and generate requisite data on toxicological effects of nanomaterials. Such data generation is especially pertinent in case of nano-dispersants, which may have the potential to cause damage to organisms in water bodies. In dealing with nanostructured materials such as CNT nanosponge, nanowire membranes etc., it is essential to ensure that individual nanotubes, nanowires etc. are not detached from the product to contaminate the water. | |
| Engineering Aspects | |
| Practical implementation of nanotechnology for oil spill remediation demands that the nanomaterials in the form of nanoparticles/ foams/sponges etc. be suitably engineered or packaged to facilitate their application. One example is TEGO, which is a powdery material that has to be contained in large porous sacks made from polypropylene or polyethylene fabric or porous film. Alternatively TEGO can be co-processed with a polymer binder in the form of a foam sheet. This open cell structure of the foam allows contact between the oil and the TEGO surfaces. The advantage of this system is that the absorbent system can be rolled for storage. Another practical example is MIT's seaswarm robot incorporating oil absorbing nanowire mesh. It is capable of autonomously navigating the surface of the ocean to collect surface oil and process it on site. Hydrophobic CF3- functionalized aerogels are integrated into devices and, for this purpose, aerogels are incorporated into solid support like fiberglass, alumina, cotton, wool carbon foam etc. This is usually done by dipping the support material in either the powdered aerogel, or in a slurry of the aerogel in a solvent, or by any other coating technique. For example, discs of fiberglass can be dipped into a solution of 15 wt % CF3- aerogel in acetone, two times and vacuum dried between dips to form a device. | |
| Cost Aspects and Commercial Viability | |
| The commercial viability of the technology is critically dependent on the cost of a product, which includes the raw material cost and the manufacturing cost. This is especially relevant in case of massive oil spills requiring huge amount of material for cleanup operation. Aerogel is one of the prime candidates for the mitigation of oil spills; however, its high cost is a major inhibiting factor for its widespread adoption. Currently, the typical cost of aerogel 16 produced by supercritical drying is about $2870 per kg while the aerogel made from discarded rice husks is reported to cost only $276 per kg, thereby making the latter a commercially viable proposition. Another interesting option is to use clay-based aerogel (Aeroclay), which uses only clay, polymer and water as raw materials; hence, its production cost is expected to be much lower than that of silica aerogels. Furthermore, it is also highly flexible in nature (unlike silica aerogel), which is a desirable property for recovering the absorbed oil by simply compressing the aerogel. | |
| Carbon nanostructured materials such as CNT sponges, VACNTs, Recam, and HRCMs are also emerging materials having great potential for oil spill cleanup and recovery on account of their outstanding properties. As these are mainly based on carbon nanotubes and graphene, economics is certain to play a major role in determining their commercial viability. Currently, MWCNTs are priced at $100-150/kg 17 and expected to reduce to $10-20/kg in the near future. The present price of graphene nano platelets is $385-525/kg and it is predicted that nanoplatelets could be produced at $11 per kilogram. Therefore, it is likely that these technologies could become commercially viable solutions for oil spill cleanup in the future. Apart from the production costs, the economic viability of a technology for oil spill cleanup also depends on other performance factors such as sorption capacity, rate of sportion of crude oil, regeneration factor, recyclability, cost of disposal of the material etc. | |
| In this context, the company Ecosorber (http://www.ecosorber.ru/) has carried out a comparative study between a conventional low cost sorbent, Peat, and Gigasorb (sorbent based on nanotechnology), to ascertain their real cost for oil spill remediation. Given the relevance of the above mentioned performance factors, the coefficient of efficiency was determined to be 0.0216 and 192.41, for peat and Gigasorb, respectively. In the case of peat, notwithstanding its low cost and ready availability, in order to collect 50 tons of spilled oil one would need 17,700 tons of peat worth $8.64 million , whereas to collect the same amount of oil only 5.6 tons of Gigasorb worth $0.131 million would be needed. Furthermore, the spent sorbents have to be recycled by an incineration process. For this purpose, the cost of inputs required for burning of the peat would be $7.05 million in addition to $8.64 million spent for the cleaning operation whereas, in the case of Gigasorb, only an additional $0.0127 million would have to be spent for burning of the sorbent. This is an apt example of how nanotechnology can help in improving the effectiveness of sorbents and, thereby, improve the economic viability of oil spill cleanup operation. | |
| Conclusions and Future Directions | |
| This article provides a general overview of the wide variety of nano-materials and -technologies that offer significant promise for oil spill cleanup and recovery. It is quite evident from the foregoing discussion that nanomaterials have enormous potential to provide innovative solutions for oil spill cleanup by virtue of their unique structure, superior properties and outstanding performance. However, for their successful implementation as commercially viable technologies, one needs to carry out a thorough study on the engineering aspects, environmental issues, scalability and cost analysis. | |
| References | |
| 1. M. Qu, J. He, J. Zhang, "Superhydrophobicity, Learn from the Lotus Leaf", Biomimetics Learning from Nature, Edited by: A. Mukherjee, ISBN 978-953-307-025-4, Publisher: InTech, March 2010 | |
| 2. J. X. Wang, Y. Z. Zhang, T. Y. Zhao, Y. L. Song, L. Jiang , "Recent research progress in wettability of colloidal crystals", Science China Chemistry, 53 (2010) 318-326 | |
| 3. L. Zhai, F. C. Cebeci, R. E. Cohen, M. F. Rubner, "Superhydrophobic Coatings", US Patent Appl. 20060029808, February 9, 2006 | |
| 4. O. Carmody, R. Frost, Y. Xi, S. Kokot, "Adsorption of Hydrocarbons on Organoclays- Implications for Oil Spill Remediation", J. Colloid and Interface Science, 305 (2007) 17-24 | |
| 5. M. O. Adebajo, R. L. Frost, J. T. Kloprogge, O. Carmody, S. Kokot, "Porous Materials for Oil Spill Cleanup: A Review of Synthesis and Absorbing Properties", J. Porous Materials, 10 (2003) 159-170 | |
| 6. J. Yuan, X. Liu, O. Akbulut, J. Hu, S. L. Suib, J. Kong, F. Stellacci, "Superwetting nanowire membranes for selective absorption", Nature Nanotechnology, 3 (2008) 332 ? 336 | |
| 7. MIT researchers unveil autonomous oil-absorbing robot, contact: Jen Hirsch, MIT News Office, http://web.mit.edu/press/2010/seaswarm.html | |
| 8. R. L. Ziolli, W.F. Jardim, "Photocatalytic decomposition of seawater-soluble crude-oil fractions using high surface area colloid nanoparticles of TiO2", Journal of Photochemistry and Photobiology A: Chemistry, 147 (2002) 205-212 | |
| 9. R. Narayan, " Titania: a material-based approach to oil spill remediation?", Materials Today, 13 (2010) 58-59 | |
| 10. S. Kwon, M. Fan, A. T. Cooper, H. Yang, "Photocatalytic Applications of Micro- and Nano-TiO2 in Environmental Engineering", Critical Reviews in Environmental Science and Technology, 38 (2008) 197 ? 226 | |
| 11. http://www.firstlinetech.com/ | |
| 12. B.C. Keller, "Nanotechnology for Spilled oil Encapsulation, remediation and Recovery", US Patent Appl. 20080249348A1, October 9, 2008. | |
| 13. J. Zou, J. Liu, A. S. Karakoti, A. Kumar, D. Joung, Q. Li, S. I. Khondaker, S. Seal, L. Zhai, "Ultralight Multiwalled Carbon Nanotube Aerogel", ACS Nano, 4 (2010) 7293-7302 | |
| 14. F.G. De Souza Jr., J. A. Marins, C. H. M. Rodrigues, J. C. Pinto, "A Magnetic Composite for Cleaning of Oil Spills on Water", Macromol. Mater. Eng., 295 (2010) 942-948 | |
| 15. I. Aglietto, "Advanced Photocatalytic Oxidation with Graphene for Wastewater Treatment", ENT Magazine, 20 March- April 2010 | |
| 16. I. Aglietto, "Combination of Materials for the Treatment of Contaminated Liquids by Means of Photocatalytic Oxidation", Int. Publ. No. WO 2010/115905 A2, Oct. 14, 2010 | |
| 15. 2020science.org | |
| 16. http://www.odemagazine.com/ | |
| 17. n.a. | |
| By Y. R. Mahajan, CKMNT. These are excerpts from the article "Nanotechnology-Based Solutions for Oil Spills". The full article with an extensive list of references appears in the January 2011 issue of Nanotech Insights. For more information, interested readers may please contact Yashwant Mahajan at [email protected] or [email protected] and obtain a copy of the full-text article in pdf format. | |
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
