| Posted: Nov 17, 2017 | |
A single-atom catalyst supported on monolayers for nitrogen fixation |
|
| (Nanowerk Spotlight) Nitrogen fixation is a process that converts nitrogen in the Earth’s atmosphere into ammonia (NH3). Nitrogen compounds are needed for the biosynthesis of basic building blocks of plants, animals and other life forms, thus nitrogen fixation is essential and crucial to sustain all forms of life. In nature, nitrogen fixation is done by the enzyme nitrogenase, and in industry, the world’s ammonia-based fertilizer is predominantly manufactured by the industrial Haber-Bosch process using H2 and N2. | |
| The main challenge of this process for NH3 synthesis is that it requires extremely high pressures (150-250 bar) and high temperatures (400-500°C) with large amounts of H2 produced by steam reforming of fossil fuels with a large concomitant emission of CO2. | |
| The electrochemical N2 reduction is rather promising for N2 fixation because it can occur at ambient temperature and pressure, in which the NH3 product can be easily separated from hydrogen feed gas, and the nitrogen reduction reaction (NRR) process can be effectively tuned by changing the operating potential, the electrolyte, the pH, etc, thus greatly improving the production yield of ammonia. | |
| Single-atom catalysts (SACs) have emerged as a new frontier in heterogeneous catalysis, and demonstrated distinguishing performances for various reactions due to their high catalytic activity with a significantly reduced amount of metals used. However, the catalytic performance of SACs for N2 fixation and conversion has been rarely explored. | |
| In new work reported in Journal of the American Chemical Society ("Single Mo atom Supported on Defective Boron Nitride Monolayer as an Efficient Electrocatalyst for Nitrogen Fixation: A Computational Study"), Prof. Jingxiang Zhao (College of Chemistry and Chemical Engineering of Harbin Normal University, China) and Prof. Zhongfang Chen (Department of Chemistry, University of Puerto Rico, USA) have proposed a quite promising single-atom-based electrocatalyst for N2 reduction to NH3 under ambient conditions. | |
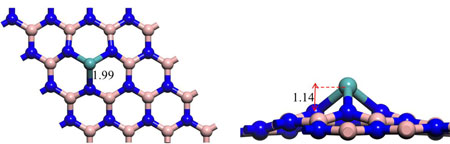 |
|
| Optimized structure of Mo-embedded BN monolayer. The unit of bond length is Å. (© American Chemical Society) | |
| "By means of density functional theory computations, we systematically evaluated the performance of a series of single transition metal atoms (Sc~Zn, Mo, Rh, Ru, Pd, and Ag) anchored on boron nitride (BN) monolayer with a B monovacancy as N2 reduction catalysts, three reaction mechanisms were explored, and the free energies of each elementary step were computed," Zhao tells Nanowerk. "We found that the single Mo atom supported by defective BN nanosheet exhibits the highest catalytic activity for N2 fixation at room temperature through an enzymatic mechanism, the overpotential is quite low, even smaller than that on the well-established Re(111) surface (0.50 V)." | |
| The team used three criteria to evaluate the performance of the catalysts: 1) the catalyst can facilitate the chemisorption of N2 molecule to guarantee the sufficient activation of its inert N-N triple bond; (2) the catalyst can selectively stabilize N2H*, and (3) destabilize NH2* species to guarantee the reduction of the overpotential. They demonstrated that Mo-embedded BN nanosheet is the only eligible electrocatalyst for the N2 electroreduction satisfying all the three screening criteria. | |
| "The large and localized spin moment as well as the decreased band gap could play a vital role in activating the N2 molecule on Mo/BN monolayer and lowering the overpotential of the whole N2 reduction. Our DFT computations showed that the reduction of NH2* group to NH3 is the potential-determining step in the most favorable enzymatic mechanism," Chen tells Nanowerk. | |
| "This study not only opens a new avenue of NH3 production by identifying promising SACs for N2 fixation at ambient conditions, but also sets up an efficient procedure in screening effective SACs based on the mechanism understanding, which can be extended to design many other SACs," he concludes. With the established screening criteria and accumulation of much larger data set, we should be able to take advantage of machine learning for the computational catalyst design in the future." | |
|
Provided by the University of Puerto Rico as a Nanowerk exclusive
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
