| Feb 14, 2020 | |
Is hematene worth pursuing? |
|
| (Nanowerk Spotlight) It is not widely known that hematite is one of the few materials useful for hydrogen production via photocatalytic water splitting. | |
| The magnetic ground state of the oxygen molecule creates restrictions on the process when other involved entities are non-magnetic, such as water and hydrogen in the water splitting reaction. | |
| Hematite as a magnetic photocatalyst can support the production of oxygen in its magnetic ground state and thereby improve the efficiency of hydrogen production. It absorbs visible light in the ultraviolet to the yellow-orange range, and its bandgap (∼2eV) is larger than the minimal energy needed to split water into hydrogen and oxygen (∼1.23eV). | |
| When hematite is made thinner, another important improvement in the efficiency of the reaction could be accomplished by expectedly more efficient separation of charge carriers upon the absorption of light on the surface. | |
| For that reason, the recent synthesis of the atomically thin, quasi two-dimensional (2D) form of hematite (Nature Nanotechnology, "Exfoliation of a non-van der Waals material from iron ore hematite") created excitement, yielding an ultrathin material with clear potential for efficient solar fuel generation. | |
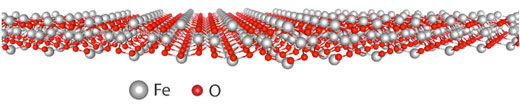 |
|
| Hematene. (Image: University of Antwerp) | |
| In their article published in 2D Materials ("Hematite at its thinnest limit"), researchers from the NANOlab Center of Excellence at the University of Antwerp now elucidate exactly how optical and magnetic properties of hematite change when decreased in thickness to the atomistic scale. | |
| Most importantly, they demonstrate that the monolayer hematite, dubbed hematene (shown in the figure above), strongly differs from the few-layer and bulk forms of hematite that exhibit very comparable properties. | |
| “Monolayer hematene promises much more efficient hydrogen prosecution than thicker hematite forms, owing to its 30% reduced band-gap – below any photon energy of visible light”, says Cihan Bacaksiz, the first author of the study. “Pristine hematene also exhibits more stable anti-ferromagnetism than thicker hematite, to temperatures well above the room temperature, and is therefore also useful for spintronic devices and dense memory concepts. Our comparative analysis to available experimental data shows that pure hematene remains evasive in the lab, but is definitely worth pursuing further.” | |
|
Source: Provided by University of Antwerp as a Nanowerk exclusive
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
