| Posted: Jan 23, 2007 | |
Self-heating nanoparticles as tumor-destroying hyperthermia agents |
|
| (Nanowerk Spotlight) Hyperthermia therapy, a form of cancer treatment with elevated temperature in the range of 41–45°C, has been recently paid considerable attention because it is expected to significantly reduce clinical side effects compared to chemotherapy and radiotherapy and can be effectively used for killing localized or deeply seated cancer tumors. | |
| Accordingly, various forms of hyperthermia have been intensively developed for the past few decades to provide cancer clinics with more effective and advanced cancer therapy techniques. However, in spite of the enormous efforts, all the hyperthermia techniques introduced so far were found to be not effective for completely treating cancer tumors. | |
| The low heating temperature owing to the heat loss through a relatively big space gap formed between targeted cells and hyperthermia agents caused by the hard to control agent transport, as well as killing healthy cells attributed to the difficulties of cell differentiations by hyperthermia agents, are considered as the main responsibilities for the undesirable achievements. | |
| In a possible breakthrough, researchers in Singapore now report the very promising and successful self-heating temperature rising characteristics of NiFe2O4 nanoparticles. Different from conventional magnetic hyperthermia, in-vivo magnetic nanoparticle hyperthermia is expected to be one of the best solutions for killing tumor cells which are deeply seated and localized inside the human body. | |
| The recent development of magnetic nanoparticle technology accelerated a new form of hyperthermia treatment, so-called “in vivo hyperthermia,” because magnetic nanoparticles are expected to provide a great deal of technical advantages for hyperthermia: 1) direct injection of hyperthermia agents through blood vessel; 2) easy transport of nanoparticles to the targeted cell by externally controlled magnetic field; 3) small heating loss during hyperthermia due to direct heating of cell; and 4) possibility for differentiation of tumor cells from healthy cells by using antibody-antigen biological reaction. | |
| However, even though the applications of magnetic nanoparticles for hyperthermia agents have been intensively studied in recent few years, there has been no distinctly successful report on the stable and saturated self-heating temperature rising and biophysiological characteristics of magnetic nanoparticles so far. Accordingly, the development of new magnetic nanoparticles, which have enhanced magnetic and biological properties for effective hyperthermia treatment, are urgently required. | |
| Dr. Seongtae Bae from the Biomagnetics Laboratory at National University of Singapore explains to Nanowerk why his team's new research findings are so significant: "We confirmed for the first time that solid state NiFe2O4 nanoparticles show very promising self-heating temperature rising characteristics, high biocompatibility, and suitable magnetic and structural properties for hyperthermia agent applications. In addition, we revealed that the maximum self-heating temperature of solid state NiFe2O4 nanoparticles is easily controlled in the biological safety and physiological tolerance ranges." | |
 |
|
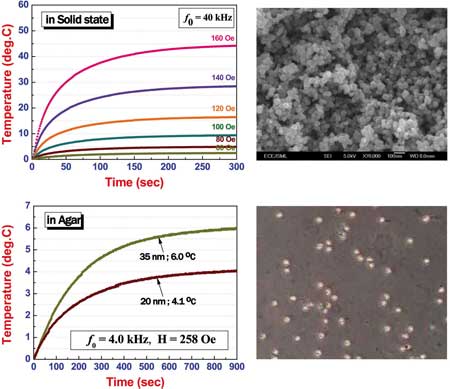
| Dependence of self-heating temperature rising characteristics of NiFe2O4 magnetic nanoparticles on applied magnetic fields at a fixed frequency in a solid and in an agar state. (Image: Dr. Seongtae Bae/NUS) | |
| By confirming that self-heating magnetic nanoparticles can be used in a controlled way and at the required temperature range of above 41°C (the required minimum temperature to kill tumor cells), Bae and his team seem to have found a promisingly designed hyperthermia agent that researchers have been looking for. | |
| These findings have been reported in recent papers in Applied Physics Letters (Applications of NiFe2O4 nanoparticles for a hyperthermia agent in biomedicine) and IEEE Transactions on Magnetics (Dependence of Frequency and Magnetic Field on Self-Heating Characteristics of NiFe2O4 Nanoparticles for Hyperthermia and Magnetic Properties, Self-Temperature Rising Characteristics, and Biocompatibility of NiFe2O4 Nanoparticles for Hyperthermia Applications). | |
| Bae points out that there are still two specific problems with the ferromagnetic nanoparticles that need to be solved. He suggests that cytotoxic problems with nickel atoms and the low temperature rising in an agar state can be overcome by coating the NiFe2O4 nanoparticles with a suitable coating material. | |
| The biocompatibility of NiFe2O4 nanoparticles was analyzed by testing the cytotoxicity of uncoated and chitosan-coated NiFe2O4 nanoparticles in agar overlays and in MTT assays. The quantitative testing results done by MTT showed that uncoated NiFe2O4 nanoparticles had a 85% cell survival rate and chitosan-coated NiFe2O4 nanoparticles had 98.8% cell survival rates. | |
| Uncoated NiFe2O4 nanoparticles in agars showed very low maximum temperature around 6°C compared to that of measured in a solid state (maximum temperature of 44.2°C) under similar self-heating measurement conditions. | |
| "We think that the extremely low self-heating temperature rising in agars possibly results from the serious changes of magnetic properties of nanoparticles adjacent to the agars such as change of spin structure of the outer shell spins induced by the chemical modification of the nanoparticle surface, change of magnetic drag force due to a viscosity variation, and reduction of magnetic particle rotation velocity" says Bae. "Considering these possible physical reasons, it can be indirectly concluded that the modification of spin structure of the outer shell of nanoparticles is closely associated with the efficiency of heat generation." | |
| In addition to hyperthermia applications, the researchers believe that their nanoparticles could be considered for an MRI contrast agent due to the particles' particularly low loss of magnetic degradations even at high frequency. | |
| Going forward, Bae describes his group's main goals regarding magnetic nanoparticle applications: "We want to develop a complete 'in-vivo hyperthermia' system using our newly developed high permeability magnetic ferrite nanoparticles. For that we need to do more research on biocompatibility and we need to develop new coating materials to improve magnetic moment and enhance the magnetic surface structure of the nanoparticles for both, an in-vivo MRI contrast agent and a hyperthermia agent." | |
| Last but not least, removing the nanoparticles from the body after treatment or MRI testing is another issue that awaits a solution. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
