| Posted: May 18, 2017 | |
Anionic regulated electrocatalysts for water oxidation |
|
| (Nanowerk Spotlight) The exhaustion of traditional fossil fuels and the growing demand for energy encourage us to develop sustainable and clean energy systems, such as fuel cells, rechargeable metal–air batteries, and water splitting devices. Oxygen evolution reaction (OER) constitutes the core process but severely limits the efficiency of the above energy devices due to its sluggish kinetics, calling for new insights into rational OER electrocatalysts design. | |
| "Improving the intrinsic reactivity of OER active sites has always been pursued, and the electronic structure of the active sites directly determines the OER performance," says Qiang Zhang, a faculty at Department of Chemical Engineering, Tsinghua University. "Cationic doping and etching are typical strategies to approach optimized electronic structures. We believe anionic regulation is another promising route to tune the electronic structure." | |
| "Metal cations constitute the actual active sites of OER reaction, while the adjacent anions regulate the electronic structure of the active sites," notes Bo-Quan Li, a Ph.D candidate of Department of Chemical Engineering, Tsinghua University. "Coupling nonpolarized anions with polarized anions is an innovative and effective strategy to tune the electronic structure and improving OER reactivity. This idea is coincidentally consistent with a Chinese traditional philosophy, 'coupling hardness and softness', where the nonpolarized anions are hard while the polarized anions are soft. Anionic regulation gives a new insight into rational OER electrocatalysts design and may also work in other catalysts system." | |
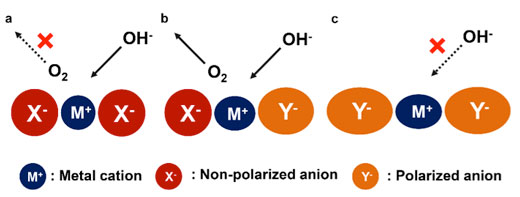 |
|
| Schematic of anionic regulation by optimizing the electronic structure of active sites toward water oxidation. a) The cations provide strong positive electric field which is favorable for the adsorption of negative hydroxyl but adverse to oxygen desorption; b) The optimized electronic structure under anionic regulation facilitates the adsorption of reactants, electron transfer, and product desorption, therefore, promotes the OER electrocatalysis; c) When the anions adjacent to the central cations are dominant polarized, the reactants cannot feel enough positive electric field and therefore the adsorption of hydroxyl is deficient. (Reprinted by permission of Wiley-VCH Verlag) | |
| In their recent paper published in Small ("Anionic Regulated NiFe (Oxy)Sulfide Electrocatalysts for Water Oxidation"), the strategy of anionic regulation was innovatively proposed and proved. | |
| "Choosing an appropriate electrocatalyst system is the first step to prove the anionic regulation strategy." This was the first thought that came to Bo-Quan’s mind after proposing anionic regulation. | |
| "Nickel and iron cations are considered as effective OER active sites in many cases. Meanwhile, oxygen anions are usually hard and nonpolarized while sulfur anions form the same group of the periodic table are soft and tend to be polarized in ionic compounds. The NiFe (Oxy)Sulfide is well worth a try." | |
| "The volcano plots of OER reactivity characterized by the overpotential at 10.0 mA cm-2 against the vulcanization degree shows the success of our anionic regulation strategy," says Qiang "and further valence analysis reveals the mechanism of the anionic regulation strategy." | |
| XPS spectra of Ni 2p demonstrate obvious shifting to lower binding energy, suggesting lower oxidative state by receiving more electrons under anionic regulation, which means the electronic structure was effectively regulated. | |
| Under the anionic regulation strategy, moderately vulcanized electrocatalyst demonstrates superb OER reactivity. The optimized NiFeS-2 electrocatalyst exhibits an ultra low overpotential of 286 mV to reach a current density of 10.0 mA cm-2. | |
| Besides, the overpotential at 10.0 mA cm-2 increases only 20 mV after 10000 s durability test. | |
| The overall water splitting performance of NiFeS-2 is also satisfactory, with the potential required to reach 10.0 mA cm-2 is 1.64 V. | |
| Optimizing the electronic structure of active sites directly determines the OER performance of electrocatalysts, and anionic regulation is proved to be sufficient for improving OER reactivity. | |
| The anionic regulation methodology not only serves as an effective strategy to construct superb OER electrocatalysts, but also enlightens a new point of view of in-depth understanding of electrocatalysis at electronic and atomic level. | |
| A Nanowerk exclusive provided by Tsinghua University | |
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
