| Posted: Apr 10, 2015 |
How many gold atoms make gold metal?
|
|
(Nanowerk News) Researchers at the Nanoscience Center at the University of Jyväskylä, Finland, have shown that dramatic changes in the electronic properties of nanometre-sized chunks of gold occur in well-defined size range. Small gold nanoclusters could be used, for instance, in short-term storage of energy or electric charge in the field of molecular electronics. Funded by the Academy of Finland, the researchers have been able to obtain new information which is important, among other things, in developing bioimaging and sensing based on metal-like clusters.
|
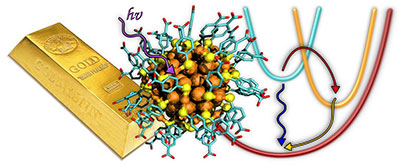 |
| Nanogold is different from macroscopic gold. The small 102-gold atom cluster (right) behaves like a giant molecule, but the slightly larger 144-gold atom cluster is like a metal. (The image on the right is from ref. 1.)
|
|
Two recent papers by the researchers at Jyväskylä (1, 2) demonstrate that the electronic properties of two different but still quite similar gold nanoclusters can be drastically different. The clusters were synthesised by chemical methods incorporating a stabilising ligand layer on their surface. The researchers found that the smaller cluster, with up to 102 gold atoms, behaves like a giant molecule while the larger one, with at least 144 gold atoms, already behaves, in principle, like a macroscopic chunk of metal, but in nanosize.
|
|
The fundamentally different behaviour of these two differently sized gold nanoclusters was demonstrated by shining a laser light onto solution samples containing the clusters and by monitoring how energy dissipates from the clusters into the surrounding solvent.
|
|
“Molecules behave drastically different from metals,” said Professor Mika Pettersson, the principal investigator of the team conducting the experiments. “The additional energy from light, absorbed by the metal-like clusters, transfers to the environment extremely rapidly, in about one hundred billionth of a second, while a molecule-like cluster is excited to a higher energy state and dissipates the energy into the environment with a rate that is at least 100 times slower. This is exactly what we saw: the 102-gold atom cluster is a giant molecule showing even a transient magnetic state while the 144-gold atom cluster is already a metal. We’ve thus managed to bracket an important size region where this fundamentally interesting change in the behaviour takes place.”
|
|
“These experimental results go together very well with what our team has seen from computational simulations on these systems,” said Professor Hannu Häkkinen, a co-author of the studies and the scientific director of the nanoscience centre. “My team predicted this kind of behaviour back in 2008–2009 when we saw big differences in the electronic structure of exactly these nanoclusters. It’s wonderful that robust spectroscopic experiments have now proved these phenomena. In fact, the metal-like 144-atom cluster is even more interesting, since we just published a theoretical paper where we saw a big enhancement of the metallic properties of just a few copper atoms mixed with gold.” (3)
|
|
The computational work was done at the CSC – the IT Center for Science in Espoo, Finland, and at the HLRS centre in Stuttgart, Germany.
|
|
Full bibliographic information
|
|
1. S. Mustalahti, P. Myllyperkiö, S. Malola, T. Lahtinen, K. Salorinne, J. Koivisto, H. Häkkinen and M. Pettersson, “Molecule-Like Photodynamics of Au102(pMBA)44 Nanocluster”, ACS Nano 9, 2328 (2015).
|
|
2. S. Mustalahti, P. Myllyperkiö, T. Lahtinen, K. Salorinne, S. Malola, J. Koivisto, H. Häkkinen and M. Pettersson, “Ultrafast Electronic Relaxation and Vibrational Cooling Dynamics of Au144(SC2H4Ph)60 Nanocluster Probed by Transient Mid-IR Spectroscopy”, J. Phys. Chem. C 118, 18233 (2014).
|
|
3. S. Malola, M.Hartman and H. Häkkinen, “Copper Induces a Core Plasmon in Intermetallic Au(144,145)-xCux(SR)60 Nanoclusters”, J. Phys. Chem. Lett. 6, 515 (2015).
|

