| Apr 07, 2020 | |
Atomic force microscopy reveals high heterogeneity in bacterial membrane vesicles(Nanowerk News) Researchers at Kanazawa University and Tsukuba University report in Nanoscale ("Diversity of physical properties of bacterial extracellular membrane vesicles revealed through atomic force microscopy phase imaging") that the physical properties of extracellular bacterial membrane vesicles are significantly diverse. The properties for a single type of bacterium as well as for different types are found to be highly heterogeneous. |
|
| One aspect of bacterial activity is the production of so-called extracellular membrane vesicles (MVs): biological ‘packages’ wrapped in a lipid-bilayer membrane, carrying for example genetic material. Apart from having specific biological functions, MVs are increasingly used in nanobiotechnological applications, including drug delivery and enzyme transport. | |
| In order to better understand the processes involving MVs, a full apprehension of their physical properties is essential. In particular, the degree of heterogeneity of vesicles released by one single type of bacterium is an important point. | |
| Now, Azuma Taoka from Kanazawa University, Nobuhiko Nomura from Tsukuba University and colleagues have addressed this question, and demonstrate a previously unrecognized physical heterogeneity in the membrane vesicles of four types of bacterium. | |
| The researchers applied phase imaging atomic force microscopy (AFM) to study the physical properties of MVs produced by E. coli, P. aeruginosa, P. denitrificans and B. subtilis. | |
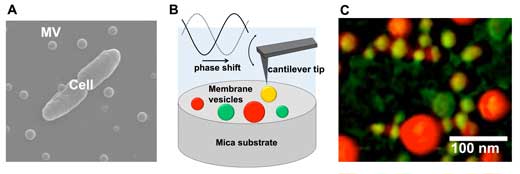 |
|
| (A) Scanning electron microscopic image of a bacterial cell and extracellular membrane vesicles (MVs). (B) Schematic drawing of MVs observation using atomic force microscopy phase imaging. (C) Mapping of MVs’ physical properties using atomic force microscopy phase imaging. MVs are color-coded on a scale ranging from “non-adherent/hard” (reddish-coloured spheres) to “adherent/soft” (greenish-coloured spheres). | |
| In phase imaging AFM, a sample is ‘tapped’ with a nano-sized oscillating cantilever tip; the observed delay in the oscillation of the tip compared to free oscillation provides a measure of the energy dissipation due to the interaction with the sample surface. This dissipation, in turn, is related to the physical properties of the surface, including adhesion, elasticity and friction, variations of which are due to compositional differences. | |
| Taoka, Nomura and colleagues recorded phase images of many MVs, and color-coded the MVs on a scale ranging from “non-adherent/hard” (low adhesion, elasticity and/or friction) to “adherent/soft” (high adhesion, elasticity and/or friction). By analyzing these maps, the scientists discovered a high diversity of physical properties of MVs. They checked whether the maps changed during imaging; the physical properties were stable in time, so the diversity could be concluded to be an intrinsic feature of MVs. | |
| The researchers found that the physical heterogeneity is caused by biological factors, as MV size and phase shifts are not correlated. Furthermore, they observed that different types of bacterium form MVs with different physical-property distributions. Finally, the scientists argued that the observed high heterogeneity reflects the chemical composition of the MVs being heterogenous. | |
| The work of Taoka, Nomura and colleagues not only presents important insights into the properties of MVs produced by different bacteria, but also shows the power of phase shift AFM as a tool for biological vesicles. | |
| Quoting the researchers: “It is expected that using these cutting-edge techniques for nanoscale physical mapping will contribute to provide further detailed information to undiscovered nature of bacterial MVs and elucidate molecular mechanisms supporting their functions.” | |
| Background | |
Phase shift atomic force microscopy |
|
| Atomic force microscopy (AFM) is an imaging technique where information is gathered by ‘touching’ the surface with a mechanical probe. The probe sits at the end of a cantilever. When the probe tip is brought near the surface, forces between tip and sample lead to a deflection of the cantilever. By measuring these deflections, a surface characterization can be obtained. | |
| In ‘tapping mode’, the cantilever is made to oscillate up and down at (or near) its resonance frequency. The forces acting on the cantilever when the tip is near the sample surface cause the amplitude of the cantilever's oscillation to change, generating a phase shift. These phase shifts provide a ‘map’ of the physical properties of the sample surface. | |
| Azuma Taoka from Kanazawa University, Nobuhiko Nomura from Tsukuba University and colleagues applied the method to study membrane vesicles. Specifically, they used high-speed AFM, which does less damage to biological samples because the tapping is ‘gentler’. | |
Membrane vesicles |
|
| Membrane vesicles (MVs) are lipid-bilayer vesicles released by bacteria. They are usually spherical in shape and have a diameter between 20 and 400 nm. The biological function of MVs is to communicate among themselves, with other microorganisms in their environment and with the host bacterium. Specifically, MVs are involved in moving bacterial cell-signaling biochemicals around. Such communication takes places in microbial cultures in oceans, inside plants, animals and the human body. The mechanism by which MVs are created is not completely understood. | |
| Taoka, Nomura and colleagues studied the physical properties of MVs by means of phase shift AFM, and revealed a high heterogeneity among MVs generated by a single type of bacterium, and further differences between different types of bacteria. |
| Source: Kanazawa University | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
