| Posted: July 8, 2008 |
Coated fullerenes might be a new hydrogen-storage material |
|
(Nanowerk News) In cooperation with their US co-workers, Chinese Academy of Sciences (CAS) researchers recently discovered a new nano-material with high-capacity hydrogen storage. Their work was published in a recent issue of Physics Review Letter.
|
|
Because of their promising prospects for hydrogen storage, carbon-based nano-materials are in the spotlight of the global community of physics over the past decade, However, the poor absorption of hydrogen molecules on the surface of the materials hinders their practical use under room temperature and pressure. To overcome the technical snag, scientists have contrived many methods to modify the carbon-based materials, including coating the surfaces with transition or alkali metals, substitutionally dope the nano-structures with light elements. Still, the resultant effects are unsatisfactory.
|
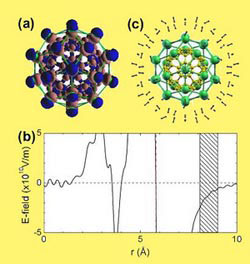 |
| Hydrogen interaction with Ca32C60 (Image: CAS)
|
|
Teaming up with ZHANG Zhenyu and his coworkers with the Oak Ridge National Laboratory, Prof. WANG Enge with the CAS Institute of Physics and his doctorate student YANG Shenyuan conducted the theoretical exploration of the feasibility to functionalize the carbon-based nanostructures in search for the characterization of their properties of hydrogen storage and concentrated their research attention on the coating of C60 fullerenes with light alkaline-earth metals.
|
|
They explored theoretically the feasibility of functionalizing carbon nanostructures for hydrogen storage, focusing on the coating of C60 fullerenes with light alkaline-earth metals. Their first-principles density functional theory studies show that both Ca and Sr can bind strongly to the C60 surface, and highly prefer monolayer coating, thereby explaining existing experimental observations. The strong binding is attributed to an intriguing charge transfer mechanism involving the empty d levels of the metal elements. The charge redistribution, in turn, gives rise to electric fields surrounding the coated fullerenes, which can now function as ideal molecular hydrogen attractors. With a hydrogen uptake of >8:4 wt%on Ca32C60, Ca is superior to all the recently suggested metal coating elements.
|
|
The research work has been funded by the CAS and the National Foundation for Natural Sciences of China in the latter's funding program specially designed for communities of innovative research.
|

