| Aug 31, 2012 |
Nobel metal nanoparticles have potential as biofuel catalysts
|
|
(Nanowerk News) Nanoparticles synthesized from noble metals such as ruthenium, rhodium, palladium, silver (Ag), osmium, iridium, platinum, and gold (Au) are attracting increased attention by researchers around the world looking for advances in such fields as biomedicine and catalysts.
|
|
Researchers from Argonne National Laboratory, the Illinois Institute of Technology, and the University of South Carolina working at U.S. Department of Energy (DOE) facilities at Argonne including the Advanced Photon Source (APS), have been successful in synthesizing and characterizing monodisperse gold-core silver-shell nanoparticles utilizing a bio-template that has potential as a water soluble catalyst for converting biomass such as dead trees, branches and tree stumps, yard clippings, wood chips, and even municipal solid waste into fuels.
|
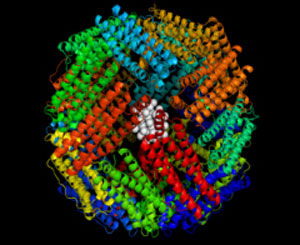 |
| Core shell nanoparticle inside Apo
|
|
Noble metals are attractive avenues for this research because, for one thing, unlike base metals, they are corrosion-resistant when exposed to damp air.
|
|
Bimetallic core-shell catalysts, where one metal is at the center, i.e., the core, and the second is at the surface, or the shell, provide distinctive properties, often a better reactivity, because the core metal particle could modify the lattice strain of the shell metal, which results in a shift of the electronic band structure of the shell metal.
|
|
Such core-shell, nanometer-sized particles are being studied in most national labs and universities.
|
|
In the field of bioinorganic chemistry, the use of protein cage templates has been recently developed as a promising method for the synthesis of uniform-size metal nanoparticle catalysts.
|
|
In this research, the protein cage template is apoferritin (Apo), which is the ferritin protein devoid of an iron core. This protein complex consists of 24 identical subunits and has a spherical shape with an outer diameter of 12 nm and an inner cavity of 8 nm, as shown in the accompanying figure.
|
|
The 8-nm cavity can be used as the location for a “nanoreactor” in which to synthesize the metal nanoparticles. The junction between the subunits consists of 14 empty channels, each 3-4 Å in diameter. These serve as a pathway between the exterior and interior of the protein core.
|
|
The metal ions, which function as the nanoreactor, diffuse into the hollow core of the Apo through these channels and subsequent reduction of metal ions in the cavity leads to one metal particle per Apo ferritin.
|
|
While the synthesis of core-shell nanoparticles has been proposed, to date there has been no report of a successful synthesis of core-shell nanoparticles inside Apo.
|
|
In a recent publication in the Journal of Materials Chemistry, the researchers in this study report for the first time synthesis of water-soluble, Apo-encapsulated, Au-core Ag-shell nanoparticles smaller than 5 nm in size and with a narrow size distribution, utilizing an unmodified Apo ("Synthesis and characterization of Au-core Ag-shell nanoparticles from unmodified apoferritin").
|
|
The particles were characterized utilizing several research techniques: small-angle x-ray scattering carried out at the X-ray Science Division beamline 12-ID of the APS; extended x-ray absorption fine structure measurements at the Materials Research Collaborative Access Team 10-ID x-ray beamline, also at the APS; scanning transmission electron microscopy done at the Argonne Electron Microscopy Center; scanning electron microscopy at the Argonne Center for Nanoscale Materials; and fast protein liquid chromatography performed at the University of South Carolina.
|
|
By carefully monitoring the amount of silver precursor, the researchers were successful in controlling the Ag shell thickness from one layer to several layers.
|
|
This method should lead the way for preparation of other core-shell nanoparticles that might function as new, potentially high-performance nanocatalysts for catalytic biofuel reactions in the future.
|
|
Such core-shell nanoparticles grown on a protein template can also be explored for future drug delivery systems.
|

