| Posted: Feb 27, 2018 | |
Light-activated black phosphorus hydrogel releases cancer drugs |
|
| (Nanowerk Spotlight) ) Earlier this year, researchers have shown that the novel concept of smart black phosphorus (BP) hydrogel could control the delivery of cancer drugs by external light and predicted that millions of patients worldwide potentially would benefit from this research (PNAS, "Novel concept of the smart NIR-light–controlled drug release of black phosphorus nanostructure for cancer therapy"). | |
| As a new member of the two-dimensional (2D) nanomaterial family, BP has attracted considerable attention in biomedicine, due to its unique physicochemical properties as well as excellent biocompatibility. | |
| BP has been used as biological imaging agent for cancer diagnosis. As a high efficiency photosensitizer, BP has been used for photothermal therapy (PTT) and photodynamic therapy (PDT). And the ultrahigh surface area endowed BP with high drug loading capacity as superior drug delivery. | |
| More importantly, the negligible cytotoxicity and great biodegradability make BP suitable for clinic applications. | |
| Now, a joint team led by Professor Han Zhang at the Shenzhen University in China, and Professor Yihai Cao at the Karolinska Institutet in Sweden, demonstrated a novel concept of light activation of BP hydrogel to release drugs for cancer therapy. | |
| This BP hydrogel is comprised of BP nanosheets (BPNSs) as a photosensitizer and hydrogel as a hydrophilic container for drugs. After injection, BPNSs convert light to thermal energy when exposed to laser irradiation, leading to heating of the hydrogel matrix. With increasing temperature, the agarose hydrogel softens to release the drug from the hydrogel scaffold into the tumor site. | |
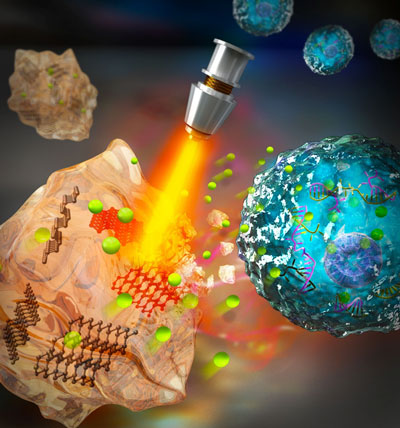 |
|
| Fig. 1: Schematic diagram of the working principle of BP@Hydrogel. BP@Hydrogel releases the encapsulated chemotherapeutics under NIR-light irradiation to break the DNA chains, leading to cell apoptosis. (Image: Dr. Meng Qiu, Shenzhen University) | |
| The process of softening is reversible and drug release rates could be accurately controlled by light intensity, exposure duration, BP concentration, and hydrogel composition. Therefore, it is possible to keep the released drugs within theur therapeutic window. | |
| After the treatment, the hydrogel further hydrolyzes and melts under enhanced laser power and finally degrades into oligomers to be excreted through urine. The waste products were found to be non-toxic. | |
| Studies on mice found that tumors treated with a DOX-loaded BP hydrogel exposed to NIR light were notably smaller, demonstrating an excellent tumor ablation effect in vivo. | |
| The BP hydrogel nanoplatform can also be loaded with other drugs to target a wide range of tumor types and other diseases, while minimizing side effects. | |
| The authors believe a design such as this has the potential to help millions of patients suffering from cancer. | |
| These findings have been published in PNAS ("Novel concept of the smart NIR-light–controlled drug release of black phosphorus nanostructure for cancer therapy"). | |
|
Provided by Shenzhen Engineering Laboratory of Phosphorene and Optoelectronics, Shenzhen University, as a Nanowerk exclusive
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
