Showing Spotlights 1249 - 1256 of 2785 in category All (newest first):
 Advances in materials, fabrication strategies and device designs for flexible and stretchable electronics and sensors make it possible to envision a not-too-distant future where ultra-thin, flexible circuits based on inorganic semiconductors can be wrapped and attached to any imaginable surface, including body parts and even internal organs. Robotic technologies will also benefit as it becomes possible to fabricate electronic skin ('e-skin') that, for instance, could allow surgical robots to interact, in a soft contacting mode, with their surroundings through touch.
Advances in materials, fabrication strategies and device designs for flexible and stretchable electronics and sensors make it possible to envision a not-too-distant future where ultra-thin, flexible circuits based on inorganic semiconductors can be wrapped and attached to any imaginable surface, including body parts and even internal organs. Robotic technologies will also benefit as it becomes possible to fabricate electronic skin ('e-skin') that, for instance, could allow surgical robots to interact, in a soft contacting mode, with their surroundings through touch.
Nov 15th, 2013
 Advances in micro- and nanoscale engineering in the medical field have led to the development of various robotic designs that one day will allow a new level of minimally invasive medicine. These micro- and nanorobots will be able to reach a targeted area, provide treatments and therapies for a desired duration, measure the effects and, at the conclusion of the treatment, be removed or degrade without causing adverse effects. Ideally, all these tasks would be automated but they could also be performed under the direct supervision and control of an external user.
Advances in micro- and nanoscale engineering in the medical field have led to the development of various robotic designs that one day will allow a new level of minimally invasive medicine. These micro- and nanorobots will be able to reach a targeted area, provide treatments and therapies for a desired duration, measure the effects and, at the conclusion of the treatment, be removed or degrade without causing adverse effects. Ideally, all these tasks would be automated but they could also be performed under the direct supervision and control of an external user.
Nov 13th, 2013
 Theranostics - a combination of the words therapeutics and diagnostics - describes a treatment platform that combines a diagnostic test with targeted therapy based on the test results, i.e. a step towards personalized medicine. Theranostic nanomedicine has the potential for simultaneous and real time monitoring of drug delivery, trafficking of drug and therapeutic responses. Researchers have now demonstrated for the first time a MRI-visual order-disorder micellar nanostructures for smart cancer theranostics.
Theranostics - a combination of the words therapeutics and diagnostics - describes a treatment platform that combines a diagnostic test with targeted therapy based on the test results, i.e. a step towards personalized medicine. Theranostic nanomedicine has the potential for simultaneous and real time monitoring of drug delivery, trafficking of drug and therapeutic responses. Researchers have now demonstrated for the first time a MRI-visual order-disorder micellar nanostructures for smart cancer theranostics.
Nov 12th, 2013
 Going hand in hand with the development of wearable electronic textiles, researchers are also pushing the development of wearable and flexible energy storage to power those e-textiles. Researchers have now developed wearable textile batteries that can be integrated with flexible solar cells and thus be recharged by solar energy. The team found unconventional materials for all of the key battery components and integrated them into a fully wearable battery.
Going hand in hand with the development of wearable electronic textiles, researchers are also pushing the development of wearable and flexible energy storage to power those e-textiles. Researchers have now developed wearable textile batteries that can be integrated with flexible solar cells and thus be recharged by solar energy. The team found unconventional materials for all of the key battery components and integrated them into a fully wearable battery.
Nov 8th, 2013
 Friction is present in numerous physical phenomena occurring at all length scale. About 1/3 of the world's primary energy is dissipated in mechanical friction and 80% of machinery components' failure is caused by wear. Friction and wear will also become bottlenecks for micro-/nano-mechanical systems (MEMS and NEMS) featured with sliding components. Superlubricity, a phenomenon where the friction almost vanishes between two solid surfaces, will be the key to solve these problems and researchers now report a breakthrough in macroscale superlubricity.
Friction is present in numerous physical phenomena occurring at all length scale. About 1/3 of the world's primary energy is dissipated in mechanical friction and 80% of machinery components' failure is caused by wear. Friction and wear will also become bottlenecks for micro-/nano-mechanical systems (MEMS and NEMS) featured with sliding components. Superlubricity, a phenomenon where the friction almost vanishes between two solid surfaces, will be the key to solve these problems and researchers now report a breakthrough in macroscale superlubricity.
Nov 7th, 2013
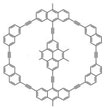 Most molecular machines operate by using chemical reactions, which lead to irreversible damage to the machine molecules themselves over time. Moreover, in most scenarios, the measurement and control of the molecular machine status are separated into distinct steps, e.g., the molecular motion is controlled by a chemical reaction, but is then detected by spectroscopy or electrochemistry. Researchers have now proposed a new type of molecular machine without chemical reactions and where the measurement/control mechanisms are combined into one.
Most molecular machines operate by using chemical reactions, which lead to irreversible damage to the machine molecules themselves over time. Moreover, in most scenarios, the measurement and control of the molecular machine status are separated into distinct steps, e.g., the molecular motion is controlled by a chemical reaction, but is then detected by spectroscopy or electrochemistry. Researchers have now proposed a new type of molecular machine without chemical reactions and where the measurement/control mechanisms are combined into one.
Nov 6th, 2013
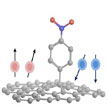 One item that so far has been missing from graphene's impressive list of physical properties is magnetism. In its pristine state, graphene exhibits no signs of the conventional magnetism usually associated with such materials as iron or nickel. So far, no reports that provide comprehensive evidence for either macro- or nanoscale magnetic phenomena for the ferromagnetism of carbon nanostructures in chemically functionalized graphene structures have appeared in the literature. Researchers have now filled this gap.
One item that so far has been missing from graphene's impressive list of physical properties is magnetism. In its pristine state, graphene exhibits no signs of the conventional magnetism usually associated with such materials as iron or nickel. So far, no reports that provide comprehensive evidence for either macro- or nanoscale magnetic phenomena for the ferromagnetism of carbon nanostructures in chemically functionalized graphene structures have appeared in the literature. Researchers have now filled this gap.
Nov 5th, 2013
 Steerable nanodevices are envisioned for a multitude of applications. For example, magnetic nanodevices can be controlled via external magnetic fields. So far, scientist mainly have used costly synthetic routes to design and synthesize such devices. Now, though, a team of scientists has shown that a very simple route based on solution chemistry can also lead to such steerable machines. So far, most nano-and microscale propeller designs have been based on a biomimetic approach. The new approach is based on random aggregates.
Steerable nanodevices are envisioned for a multitude of applications. For example, magnetic nanodevices can be controlled via external magnetic fields. So far, scientist mainly have used costly synthetic routes to design and synthesize such devices. Now, though, a team of scientists has shown that a very simple route based on solution chemistry can also lead to such steerable machines. So far, most nano-and microscale propeller designs have been based on a biomimetic approach. The new approach is based on random aggregates.
Nov 4th, 2013
 Advances in materials, fabrication strategies and device designs for flexible and stretchable electronics and sensors make it possible to envision a not-too-distant future where ultra-thin, flexible circuits based on inorganic semiconductors can be wrapped and attached to any imaginable surface, including body parts and even internal organs. Robotic technologies will also benefit as it becomes possible to fabricate electronic skin ('e-skin') that, for instance, could allow surgical robots to interact, in a soft contacting mode, with their surroundings through touch.
Advances in materials, fabrication strategies and device designs for flexible and stretchable electronics and sensors make it possible to envision a not-too-distant future where ultra-thin, flexible circuits based on inorganic semiconductors can be wrapped and attached to any imaginable surface, including body parts and even internal organs. Robotic technologies will also benefit as it becomes possible to fabricate electronic skin ('e-skin') that, for instance, could allow surgical robots to interact, in a soft contacting mode, with their surroundings through touch.
 Subscribe to our Nanotechnology Spotlight feed
Subscribe to our Nanotechnology Spotlight feed





