| Posted: Jan 02, 2013 | |
Unique mussel wet-adhesion improves lithium-ion battery performance |
|
| (Nanowerk Spotlight) Binders are used in fabricating lithium-ion batteries to hold the active material particles together and in contact with the current collectors. The characteristics of the binder material used are critical for the performance of the battery. | |
| The anode is a critical component for storing energy in lithium-ion batteries. Silicon has recently attracted considerable attention as an anode material in lithium battery technology due to its unparalleled capacity, which is about ten-fold higher than those of the conventional graphite anodes. Despite the excellent capacity, however, silicon suffers from short cycle life. The cycle life of silicon is typically less than a couple hundred of charge-discharge cycles limiting its application. | |
| The reason for the limited cycle life is poor film stability because silicon – during its reaction with lithium ions – undergoes a very large volume expansion by up to 300% during charge and discharge. | |
| And this is where the binders come into play. To minimize the side effects of the large volume expansion, the binders included in the electrode films (both cathodes and anodes) play a critical role in maintaining stable electrode structures over a large number of cycles. Although intensive research related to binders has been performed, the success has been limited. | |
| In an effort to make a highly functional binder, researchers at the Korea Advanced Institute of Science and Technology (KAIST), led by associate professors Jang Wook Choi and Haeshin Lee, have developed polymers conjugated with mussel-inspired functional groups (catechol groups). Catechol was found to play a decisive role in the exceptional wetness-resistant adhesion. | |
| The results have been published in the [date] online edition of Advanced Materials ("Mussel-inspired Adhesive Binders for High Performance Silicon Nanoparticle Anodes in Li-Ion Batteries"), first-authored by Myung-Hyun Ryou. The results suggests that the binder plays a critical role in the operation of pure silicon and silicon-graphite composite anodes. | |
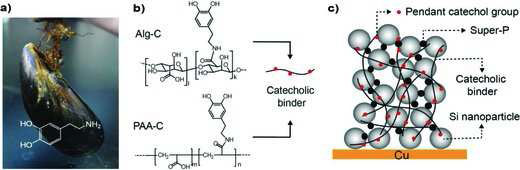 |
|
| Catechol conjugated polymer binders and Si anode structure. a) Mussel; the inset shows the chemical structure of dopamine inspired from mussel foot proteins. b) Structural formula of Alg-C and PAA-C alongside a simplified structure of a conjugated polymer binder; the black solid line represents the polymer backbone with carboxylic acid functional groups attached and red circles represent catecholmoieties conjugated to the backbone. c) A graphical illustration of the Si NP anode structure. (Reprinted with permission from Wiley-VCH Verlag | |
| Due to significantly enhanced adhesion, the silicon electrodes become much more stable, enabling to improve their cycle lives significantly, i.e. several times compared to previous polymeric binders. | |
| Mussel feet show exceptionally strong holdfast on wet surfaces. They preserve strong adhesion on the rock surfaces even under fierce sea wave action. Such exceptional wet-adhesion is nowadays giving clever ideas for making breakthrough progress in a variety of technological areas. For instance, researchers have used "mussel glue" to fabricate DNA chips for diagnostics and research or generally used the adhesive properties of mussels to develop new adhesive materials. | |
| "Although the battery community has noticed the importance of polymeric binders in the emerging silicon battery anodes, previous binders have shown limited success," Choi tells Nanowerk. "In comparison, the mussel-inspired binders that we used in our work enhance the electrode film stability remarkably and thus improve the cycle life." | |
| He points out that the wetness-resistant adhesion found in mussel glue could be very useful for battery operations because the battery components are also in contact with each other in liquid environments. | |
| Choi notes that, having taken note of the recent studies that indicated the importance of the rigidity of polymer backbone in retaining the capacity of the silicon electrodes during cycling, the team conjugated adhesive catechol functional groups to well-known poly(acrylic acid) (PAA) and alginate backbones with high Young?s moduli. | |
| "As for the morphology of the active material, among various silicon nanostructures, we chose silicon nanoparticles on account of their capability for mass production," he says. "The mussel-inspired binders endow silicon nanoparticles electrodes with markedly improved battery performance compared to those based on other existing binders." | |
| Silicon has already been partially included as an anode material in certain battery applications and is expected to increase its presence in future applications. Thus, the binder developed by the KAIST team would accelerate and expand the use of silicon in future lithium-ion batteries. | |
| "For delivery of the mussel-inspired binders into real markets, further electrode optimization might be required perhaps under collaborations with battery industries," says Choi. "Moreover, this mussel-inspired binder should be readily applicable to other lithium-ion battery electrodes that undergo significant volume change during cycling because the wetness resistant catecholic adhesion proved to be effective with various substrates." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
