| Posted: Jun 04, 2014 | |
A chronic in-vitro model for assessing the long-term bioeffects of nanomaterials |
|
| (Nanowerk Spotlight) Studying the adverse effects of engineered nanomaterials on living organisms and the ecosystems is a daunting task, given the sheer number of nanomaterials that require testing. In vitro models, which due to time and cost restraints associated with animal models constitute the majority of studies on the safety of nanomaterials to regularly identify nanomaterial-induced biological effects. Besides cytotoxicity, these include augmented cellular stress, DNA damage, secretion of pro-inflammatory and immune markers, indicating a high potential for long-term health concerns. | |
| "There exists serious conflict with correlating acute, 24-hour in vitro nanomaterial exposure findings to a chronic in vivo model, illuminating the critical need for a new approach to feasibly and reliably assess nanomaterial consequences in cell culture," Saber M. Hussain, Senior Scientist at the Air Force Research Laboratory and a Professor of Pharmacology & Toxicology at Wright State University, tells Nanowerk. | |
| Numerous nanotoxicological studies reporting effects of nanomaterials typically address a single exposure at high dosages that are irrelevant to realistic human exposure. | |
| Recognizing that acute in vitro work had extremely low correlation to in vivo nanomaterial studies, coupled with the recognition that the unique characteristics that distinguish nanomaterials vary as a function of time, Hussain and his team sought to identify a model that would allow for the evaluation of nanomaterial behavior over a 3-month period, but be carried out in an in vitro model. | |
| They now have reported the findings of their study in ACS Nano ("Less Is More: Long-Term in Vitro Exposure to Low Levels of Silver Nanoparticles Provides New Insights for Nanomaterial Evaluation"). | |
 |
|
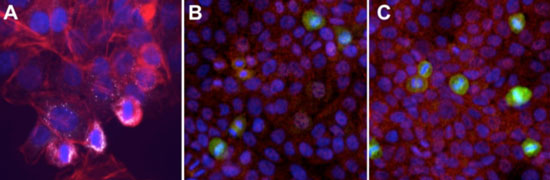
| This figure demonstrates that A) even at the low dosages used for daily exposure (400 pg/mL) the silver nanoparticles were able to be retained by the HaCaT cells. Nanoparticles are clearly visible (reflecting white) amidst the actin (red) and nuclear (blue) staining of chronically dosed cells. Images B (acute dosing) and C (chronic dosing) show how the stress response, as evaluated through p38 fluorescent imaging (green), is of a higher degree for the chronically exposed cells. (Image: Prof. Hussain, Wright State University) | |
| This work is unique with respect to the fact that current nanotoxicological investigations are either acute in vitro or chronic in vivo. By designing and implementing a chronic in vitro model for nanomaterials' bioeffects it seamlessly merges the advantages of both in vitro – including lower cost, ability for high throughput, and no ethical concerns regarding animal models – with the improved predictive capabilities and long term data associated with chronic models. | |
| "We believe that the advantages of the in vitro model, such as cost effectiveness, cell line specificity, an in-depth mechanistic evaluation allow for greater flexibility and targeting in experimental design," says Hussain. | |
| The unique aspect of this study is that the researchers demonstrated a proof of concept in which skin cells were chronically exposed to silver nanoparticles at low dosages (in the pg/mL range) for 8 hours a day, five days a week, for 14 consecutive weeks. The biological responses of the chronically dosed cells were then compared against a one-time acute exposure. | |
| The results demonstrated that chronic exposure induced elevated stress proteins, produced gene changes, and altered basic cell functionality. | |
| "These finding indicate that the biological effects of silver nanoparticles are based on not only dose but also on exposure duration," notes Hussain. "In conclusion, our data demonstrated that incorporating chronic exposure at realistic dosages is essential to understanding nanomaterials long term biological effects that cannot be detected for acute exposure scenarios." | |
| He points out that a long-term goals of this work would be to develop enhanced in vitro models that more closely behave as an in vivo system and allow for better predictive capabilities regarding nanomaterial effects. | |
| "As the continued generation of new classes of nanomaterials require accurate tools for their safety evaluation, it is critical that accurate cellular models are in place to elucidate their cellular interactions and induced bioeffects," he says. | |
| Potential applications of this work include the standardization of a chronic in vitro model for nanomaterial safety assessments and potential evaluation of nanomaterial standards as they are generated. | |
| Hussain and his team hope that this work also gets other scientists thinking and exploring how they can incorporate in vitro exposures of extended durations to better quantify toxicological responses. | |
| Given the success of this initial pilot experiment, the scientists now plan on exploring other core nanomaterials to ascertain if any other differential responses are identifiable. | |
| "We are very eager to expand to alternate cell lines that would align with the route of exposure for these other select nanomaterials, such as a lung, liver or brain model, or even a co-culture," says Hussain. "As with this proof of concept study, many challenges exist, primarily in the design an optimization associated with incorporating a new cell line and nanomaterials. Also, the best selection of nanomaterial dosages and what dosimetry metric to use is still a very hot topic at the moment." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
