| Posted: Apr 03, 2008 | |
Extremely sensitive protein detection with quantum dot self-assemblies |
|
| (Nanowerk Spotlight) In proteomics research, the study of the structure and function of proteins, chemical as well as physical methods are used to detect proteins. Physical methods are mostly applied after chromatography. They are either based on spectroscopy like light absorption at certain wavelengths or mass determination of peptides and their fragments with mass spectrometry. Chemical methods are used after two-dimensional electrophoresis and employ staining with organic dyes, metal chelates, fluorescent dyes, complexing with silver, or pre-labeling with fluorophores. What these various methods have in common is that they are not very fast, can be expensive, sometimes don't offer the sensitivity required, and are not always easy to handle. Since protein detection can be a powerful tool for diagnosing, prognosing, and monitoring cancers and other medical conditions, researchers are working towards developing detection platforms that can multiple specific molecules from the complex mixture present in serum, and is rapid, sensitive, and simple to administer. | |
| When a malignant cancer develops in the human body, the cancer cells produce certain types of proteins. Identifying these proteins enables early detection of cancer. One of the goals of nanobiotechnology is to develop protein chips that are sensitively responsive to a very tiny amount of specific proteins in order to enable early stage diagnosis. For example, a protein that is known to bind to a protein produced by a cancer cell could be attached to a biochip. If this particular cancer cell protein were present in a sample passed over the chip, it would bind to the protein on the chip, causing a detectable change in the electrical signal passing through the chip. | |
| Researchers now have demonstrated a simple and rapid way of detecting proteins of interest using nanoparticles. This single step reaction starts with nanoparticle-antibody conjugates that form large aggregates if the intended protein molecules are present in the solution. The large aggregates can be characterized individually by laser scattering and fluorescence. | |
| "Compared to traditional proteomic detection techniques, antigen mediated quantum dot agglomeration combined with flow-based detection on a microfluidic device has the potential to offer better sensitivity, ease of use, speed, and cost of testing" Dr. Todd D. Giorgio tells Nanowerk. "We have developed a novel antigen detection technique based on fundamental nanoscale phenomena. This technique has several advantages over conventional antigen detection strategies due to the use of solution-phase biofunctionalized quantum dots and a microfluidics-based detection strategy. We carried out sensitive detection completely in the fluid phase, in a single step, and with minimal incubation." | |
| Giorgio, a Professor of Biomedical Engineering and Professor of Chemical Engineering at Vanderbilt University in Nashville, Tennessee, and Chinmay P. Soman, a student in his group, have published a paper in Langmuir that describes their novel approach to to sensitive and rapid antigen detection ("Quantum Dot Self-Assembly for Protein Detection with Sub-Picomolar Sensitivity"). | |
| "Our paper demonstrates a novel and efficient method for detecting low concentration proteins, which could be used for early diagnosis and frequent monitoring of diseases such as cancer using just small volume blood sample" says Soman, the paper's first author. | |
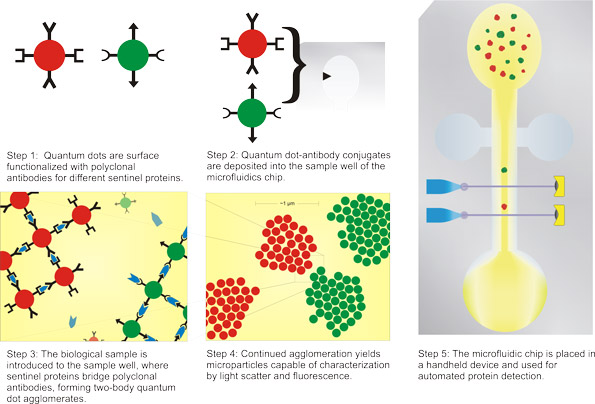 |
|
| The graphic depicts a possible implementation of this technology for improved ease of use, consisting of a microfluidic chip and a dedicated hand held or benchtop device. (Image: Daniel Dorset) | |
| While conventional protein detection methods are time consuming and labor intensive, the method developed by the Vanderbilt researchers could be automated to reduce time and cost. Giorgio says that, compared to other nanoparticle-based protein detection methods, their approach offers a better potential to detect multiple proteins simultaneously and quantitatively. | |
| Another advantage is that the technique requires only very small volumes of samples – it may be possible to detect proteins from a small blood sample. The antigens in this study were detected to sub-picomolar concentration, comparable to the detection of the same molecules by conventional techniques such as ELISA or Western blot. | |
| "Our agglomeration-based detection strategy is already comparable to conventional immunorecognition techniques in terms of sensitivity" says Giorgio. "The flow-based detection approach also enables characterization of multiple properties of individual self-assembled structures, thus providing a tool for investigating self-assembly in a novel and powerful manner, which may lead to insights into this important nanoscale phenomenon." | |
| Soman explains that for their technique, quantum dots are conjugated with polyclonal antibodies using a streptavidin-biotin interaction. In the presence of the appropriate antigen molecules, these quantum dot/antibody conjugates rapidly self-assemble into colloidal structures with sizes that are 1-2 orders of magnitude larger than the constituents. "The size, structure, and fluorescence characteristics of these self-assembled structures are a function of the relative concentration of the conjugates and the antigen molecules, among other factors" he says. "These attributes of the colloidal structures can be characterized by several techniques, including flow cytometry, dynamic light scattering, and electrical sensing zone or Coulter counter method. | |
| Non-specific aggregation of nanoparticles can be a problem when a lot of proteins are present in the sample, such as in serum. This is a well known problem when nanoparticles and biological materials interact, and it is especially important for applications such as this one that depend on predictable size of the nanoparticles over time. Giorgio and Soman show that this problem can be solved by effective surface engineering of the nanoparticles to minimize non-specific interactions. | |
| The scientists say that they managed to separately detect two different proteins with high sensitivity and specificity. Currently, they are investigating simultaneous detection of multiple proteins from a complex mixture such as a serum. | |
| "Our technique requires minimal sample volume, is amenable to multiplexing, automation, and implementation in a microfluidic chip, and is completely modular," says Giorgio. "This makes it an ideal candidate as a platform technology for frequent and low cost proteomic testing and monitoring of cancers, as well as an advanced point-of-care diagnostic based on a diverse array of intermolecular recognition-based biomarker sensing.". | |
| The implementation of this and other protein detection techniques on microfluidic and/or semiconductor chips (lab-on-a-chip) is expected to make diagnostics simpler, faster and cheaper, leading to better therapy effectiveness and survival rates for serious diseases. However, the maturation of proteomics into a clinically proven technology will be required before these compact diagnostic devices can be commercialized. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
