| Posted: Oct 07, 2009 | |
Nanotechnology therapy for brain cancer |
|
| (Nanowerk Spotlight) Here is a no-brainer (excuse the pun): If you had brain tumor, would you rather receive your medicine through an injection in the arm or have a hole drilled in your skull? Even if you opted for the 'hole-in-the-skull' method, brain cancers are often inoperable due to their location within critical brain regions or because they are too small to detect. Nanotechnology offers a vision for a smart drug approach to fighting tumors: the ability of nanoparticles to locate cancer cells and destroy them with single-cell precision. One of the most important applications for such nanoparticulate drug delivery could be the delivery of the drug payload into the brain. However, crossing the brains protective shield, the blood-brain barrier, is a considerable challenge. Novel targeted nanomedicine drug delivery systems that are able to cross this barrier bring us closer to this vision of brain cancer destroying drugs. | |
| The utter scarcity of techniques for brain-specific delivery of therapeutic molecules using non-invasive approaches has led researchers to increasingly explore the vast potential of nanotechnology toward the diagnosis and treatment of diseases/disorders incurable with present techniques. | |
| In an advance toward better treatments for the most serious form of brain cancer, scientists have now developed a nanoparticle-based therapy that seek out and destroy brain cancer cells without damaging nearby healthy cells. | |
| Reporting their findings a recent issue of Nano Letters ("A High-Performance Nanobio Photocatalyst for Targeted Brain Cancer Therapy") a team of scientists at Argonne National Laboratory show the first evidence of successful bioconjugated nanoparticles targeting toward cancer and away from normal brain cells. | |
| The researchers made use of the fact that titanium dioxide nanoparticles exhibit cytotoxicity toward some tumors. The paper's first author, Elena Rozhkova from Argonne's NanoBio Interfaces group, writes that "although nanomaterials tend to passively accumulate in tumors due to the so-called enhanced 'permeability and retention effect' and often serve as nanocarriers for chemotherapeutics, this passive strategy has limitations because of its random delivery mode." | |
| In this new work, the Argonne team proposes a technique to overcome the passive transport drawbacks by integrating the hard inorganic nanomaterial with a biological soft material, an antibody that is able to recognize glioblastoma multiforme (GBM) cells, a malignant glioma that represents a devastating form of primary brain cancer characterized by resistance to conventional adjuvant therapies. | |
 |
|
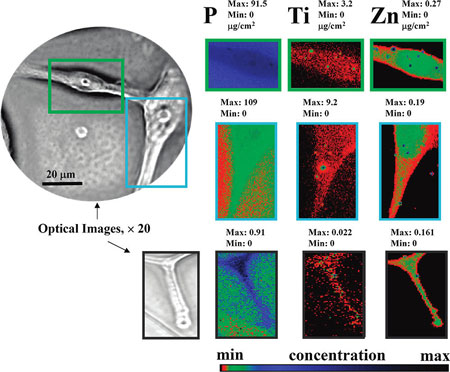
| XFM-based visualization of the TiO2-mAb binding to the single GBM cells. Elemental distribution of biogenic phosphorus and zinc are used to sketch cells and nucleus. Directed by the mAb exclusively to the surface of GB cells, TiO2 nanoparticles are spread all through the GMB cell, including cells invadopodia (lower images). The intensity of the elemental images was displayed using a prism color table in logarithm scale, which is shown in the bottom right. The max and minimum threshold values in micrograms per squared centimeter are given above each frame. Scans were obtained by using 10.0-keV incident energy with dwell times of 1 s per pixel and 1-µm steps through the sample. The simultaneous appearance of intense “hot spots” in the Ti and Zn distribution images are possibly the result of the titanium nanoparticles as well as other inorganic materials aggregation. (Reprinted with permission from American Chemical Society) | |
| Like photodynamic therapy, this approach includes three main components: light, oxygen, and a photoreactive material. | |
| The delivery platform developed by Rozhkova and her colleagues uses 5 nm titanium dioxide nanoparticles that are covalently conjugated with an antibody that specifically targets certain tumors, including GBM. A naturally occurring metabolite of dopamin, DOPAC, is used as a linker molecule to tether the antibody to the nanoparticles. | |
| The whole thing works like this: the titanium dioxide/antibody nanobiocomposite binds exclusively to GBM cells. The hybrid semiconductor particles absorb energy from light, which is then transferred to molecular oxygen, producing cytotoxic reactive oxygen species (ROS). ROS damages the cell membrane and induces programmed death of the cancer cell. | |
| One drawback of this method is that brain tumors can not be exposed to light directly, but even the deepest brain tumors may become accessible during surgery, and light-based techniques may serve as an excellent intraoperative adjuvant therapy. So, for this particular technique, you still would need to drill a small hole in the skull, if only to insert a probe with a light-emitter at its end. | |
| Another significant finding of this work is that, for first time, the researchers report the direct visualization of ligand-receptor interactions and mapping of a specific human GBM receptor through a single brain cancer cell using titanium dioxide nanoparticles through XFM hard X-rays of Argonne's Advanced Photon Source. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
