| Posted: Apr 07, 2014 | |
New synthesis method for graphene using agricultural waste |
|
| (Nanowerk Spotlight) The four major synthesis methods for producing graphene are: chemical vapor deposition (CVD) on metallic films, the epitaxial growth on silicon carbide, liquid exfoliation of graphite crystals, and the chemical reduction of graphene oxide. | |
| Using these processes it is possible to produce high-quality and large-sized graphene in large quantities. However, they all come with drawbacks as well: the complexity of the CVD process – including high temperatures and expensive substrates – makes it not very suitable for bulk production; epitaxial growth provides a wafer-scale graphene, but silicon carbide is expensive and this process requires high temperature above 1500°C; and exfoliation or chemical reduction of graphite oxides can produce graphene in a scalable manner although the use of toxic chemical agents as well as complex processing requirements tends to complicate the scaling up of such processes. | |
| In new work, researchers have now proposed an alternative way of making graphene from rice husk. They reported their findings in the March 27, 2014 online edition of Small ("Rice Husk-Derived Graphene with Nano-Sized Domains and Clean Edges") | |
 |
|
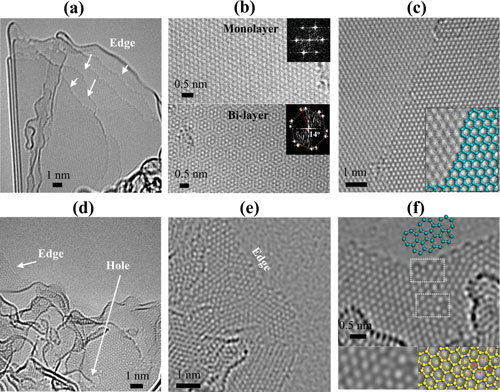
| Atomic-scale TEM images of crystalline micro-sized graphene: (a) crystalline-graphene with an exposed edge-rich structure; (b) monolayer graphene (upper) and bilayer graphene exhibiting 14° stacking rotation (bottom); (c) AA stacking exhibiting zigzag edges. Atomic-scale TEM image of corrugated graphene: (d) corrugated graphene exhibiting an edge- and nanopore-enriched structure; (e) clean edges with domains a few square nanometers; (f) localized topological defects in the edges and hexagonal carbon lattices. Note that the five- and seven-membered rings are clearly shown. (Reprinted with permission from Wiley-VCH Verlag) (click image to enlarge) | |
| This research, using an ordinary synthetic apparatus and abundant agricultural waste, suggest that low cost graphene materials could now be easily and cheaply synthesized on an industrial scale. | |
| The annual global rice production reaches almost 700 hundred million tons, and a part of the waste from rice husks – the outer, protective covering of a rice kernel which is about 20 wt% of the entire kernel, or roughly 120 million tons a year – can be a massive resource as feed material for graphene production. | |
| Due to its abundance, risk husk has already received much attention as a starting material in generating high-value-added materials such as silica and porous carbon. For instance, in a previous Nanowerk Spotlight we reported on the use of rice husks for the production of silicon with an ideal porous nanostructure for use in high-capacity lithium-ion battery anodes (read more: "Will future battery parts be grown on a rice field?"). | |
| "In our recent research we demonstrated the ability of synthesizing bulk amounts of crystalline graphene with nanosized domains in a rapid, reliable, scalable, and cost-effective manner, by chemically activating (with potassium hydroxide) agricultural waste such as rice husk ash," Hiroyuki Muramatsu, an Assistant Professor in the Faculty of Engineering at Shinshu University in Japan, Yoong Ahm Kim, an Associate Professor in School of Polymer Science and Engineering at Chonnam National University in Korea, tell Nanowerk. | |
| Although the production of activated carbon from rice husk ash has a long tradition, this is the first time that graphene structures have been observed in rice husk-derived activated carbon. | |
| "We have also noted for the first time that highly crystalline and atomically clean edges are present in the synthesized materials, even though our graphene sample is prepared at relatively a low temperature (850°C)," Muramatsu and Kim point out. | |
| The nano-sized crystalline graphene exhibited a monolayer or multilayer structure with clean edges, whereas the corrugated graphene consisted of domains a few nanometers in size (200-300 carbon atoms), showing clear grain boundaries. | |
| "Our findings clearly demonstrate that rice husk ash could be converted to high-value-added graphene in a rapid, reliable, scalable, and cost-effective manner," say Muramatsu and Kim. | |
| They add: "The presence of clean and stable edges might possess novel physicochemical properties that make our material very attractive when fabricating high-performance carbon-based energy storage and conversion devices – e.g., supercapacitor and hydrogen storage – next-generation water filter and various nanocomposites." | |
| According to the researchers, there are still some issues to resolve. First, the used process (calcination and KOH treatment) makes it difficult to assess the detailed growth mechanism of graphene with its unique structures. Secondly, the production process needs potassium hydroxide treatment to synthesize graphene. The team is keen to find an alternative activation process without using strong alkali compounds. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
