| Posted: May 14, 2014 | |
Understanding what happens at the interface of enzymes and nanomaterials |
|
| (Nanowerk Spotlight) Studies have shown that in a favorable nano environment, enzyme immobilization onto nanosupports could lead to increased enzyme stability and improved specificity, and could allow for prolonged enzyme functionality through chemical (e.g., cross-linking) and physical treatment (e.g., pH enhancement or lyophilisation). Researchers also have shown that immobilization onto carbon-based nanosupports can increase the enzyme turnover and allow for prolonged enzyme-based conjugates isolation and usage. | |
| However, the molecular mechanisms and synergistic reactions that take place at the nanosupport interface upon enzyme immobilization have yet to be fully understood and exploited for future advances in enzyme-based applications to be made possible. | |
| In new work, researchers have now taken another step towards the detailed characterization and optimization of enzyme-nanosupport interface reactions. In a paper in a recent issue of ACS Applied Materials & Interfaces ("Enzyme Catalytic Efficiency: A Function of Bio–Nano Interface Reactions"), they present a systematic study of the interplay reactions that take place upon immobilization of three pure enzymes namely soybean peroxidase, chloroperoxidase, and glucose oxidase at carbon-based nanosupport interfaces. | |
 |
|
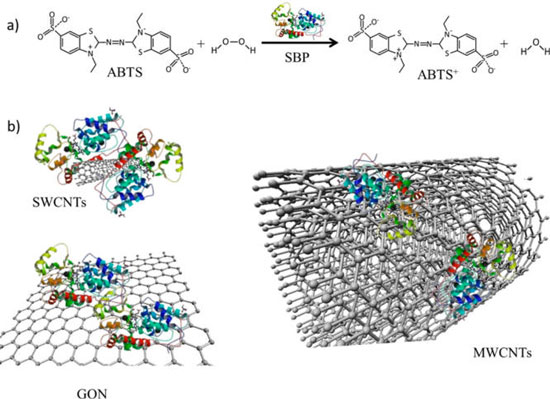
| Concept schematic of soybean peroxidase (SPB) immobilization onto carbon-based materials. (a) SBP catalyzes the oxidation of ABTS to ABTS+. (b) SPB immobilization onto carbon-based materials with different surface curvatures and aspect ratios led to enzyme-based conjugates. (Reprinted with permission from American Chemical Society) | |
| "I believe that by actively 'interrogating' the interface between the biological molecules and nanomaterials, we could possibly be able to track biofunctionality as an adaptive behavior-function of the individual components simply based on their reactions and thermodynamics upon blending/ interfacing," Cerasela Zoica Dinu, an assistant professor in the Department of Chemical Engineering, Benjamin M. Statler College of Engineering and Mineral Resources, West Virginia University, tells Nanowerk. "Intelligent design and control of the bionano interface could ensure functionality, high operational stability, efficiency, yield of recovery and conversion, and reduced enzyme inhibition for instance making the myriad of enzyme-based technologies a daily reality." | |
| The team's systematic studies have shown that fine control of the symbiotic interplay between enzyme and nanosupport characteristics can lead to enhanced catalytic efficiency at nanointerfaces. | |
| In particular, the researchers showed that the activity and efficiency of the enzyme/carbon-based conjugates could be tuned by the user by controlling the enzyme immobilization conditions, and the local properties and characteristics of the nanosupport. | |
| Dinu explains that understanding and exploiting the interface between biological molecules and synthetic nanomaterials could potentially lead to a novel class of complex, functional bionanotechnology tools. | |
| "With these new tools, the user could exploit the self-assembly, self-recognition, specificity and high efficiency of biology, combined with the exceptional chemical, electrical, and mechanical properties of nanomaterials," she says. " | |
| Systematic studies like this, trying to reveal the underlying mechanisms that control enzyme activity and catalytic behavior at nanointerfaces, seek to unravel whether there is an optimum support to be used for a specific enzyme immobilization in order to lead to maximum catalytic efficiency of that enzyme. | |
| By combining relational and conceptual design and building on prior knowledge of the biocatalyst/nanomaterial characteristics one could design adaptive strategies in which the responses account for maximum retained enzyme activity while augmenting recovery of the active enzyme-nanomaterial conjugates. | |
| "For instance" says Dinu, "one could envision the ability to build a comprehensive and accurate database in which the nanomaterials and enzymes 'repertoires' are interfaced to ensure optimum functionality and efficiency. By rationally designing 'adaptive' biomolecular sensors able to use high catalytic activity and selectivity one could provide analytics-predictive responses in real-time. Such a 'catered to fit' approach could lead to efficient, miniaturized, lower-power and highly specific sensors to be further used for biodiagnosis or environmental and biomedical monitoring or energy storage, just to name a few. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
