| Posted: Sep 04, 2014 | |
Titania nanotubes' role in building ultrafast rechargeable Li-ion batteries |
|
| (Nanowerk Spotlight) Nanotechnology has the potential to deliver the next generation lithium-ion batteries (LIBs) with improved performance, durability and safety at an acceptable cost (read our comprehensive overview here: "The promise of nanotechnology for the next generation of lithium-ion batteries"). For instance, faster intercalation of lithium ions can be facilitated by using nanosized materials for electrodes, which offer high surface areas and short diffusion paths, and hence faster storage and delivery of energy. | |
| During the charging process of lithium-ion batteries, lithium ions migrate from cathode to anode, where they become embedded in the porous electrode material in a process known as intercalation. | |
| These electrodes are typically made from graphite. When the battery is used, the ions migrate back to the cathode, sending electrons through the circuit. However, graphite has a fairly low storage capacity and release rate, so finding alternatives is key to making batteries that last longer, produce more power, and charge much faster. | |
| "Several challenging bottlenecks remain to build the ideal nanostructured electrodes for ultrafast rechargeable LIBs," Xiaodong Chen, an associate professor in the School of Materials Science & Engineering at Nanyang Technological University, explains to Nanowerk. "For instance, the particulate nanomaterials tend to aggregate during cycling, resulting in the formation of poor conduction network and loss of particle connectivity. This increases the resistance between the electrode and current collector after long-time cycling." | |
| To overcome these shortcomings, Chen and his team hypothesized that is possible to develop a robust three-dimensional network architecture with anti-aggregation property for long-time cycling through the assembly of continuous one-dimensional titania nanotubes, which provides direct and rapid ion/electron transport pathways and adequate electrode-electrolyte contact as well as short lithium ion diffusion distance compared with other nanostructures. | |
| As the team reports in two new papers in the July 10, 2014 online edition of Advanced Materials ("Mechanical Force-Driven Growth of Elongated Bending TiO2-based Nanotubular Materials for Ultrafast Rechargeable Lithium Ion Batteries") and the August 28, 2014 online edition of Angewandte Chemie International Edition ("Unravelling the Correlation between the Aspect Ratio of Nanotubular Structures and Their Electrochemical Performance To Achieve High-Rate and Long-Life Lithium-Ion Batteries"), they succeeded in developing a mechanical force-driven method to prepare elongated bending TiO2-based nanotubes for high-rate LIBs. | |
 |
|
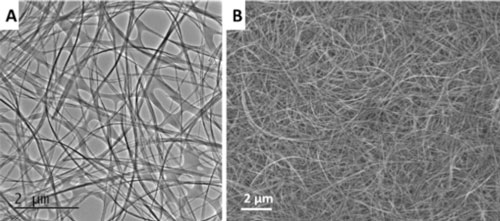
| (a) TEM and (b) SEM images of the elongated TiO2-based nanotube formed by stirring hydrothermal method by Prof. Chen's team (Image: Chen group) | |
| "We found that diffusion-limited reaction in traditional hydrothermal growth could be systematically manipulated by mechanical stirring, which we used to control the aspect ratio of viscous titanate nanotubes," Chen tells Nanowerk. "We used these elongated nanotubes with length up to tens of micrometers to fabricate additive-free TiO2-based electrode materials." | |
| He notes that, traditionally, the hydrothermal technique is conducted in static conditions and the diffusion-limited reaction may limit the growth kinetic of nanotubes. The stirring process is commonly used during materials synthesis for homogeneous mixing of reactants in solution, and to increase the reaction rate. | |
| In order to integrate a stirring process into the traditional hydrothermal reaction method, Chen's lab built a homemade stirring hydrothermal system to prove their concept idea. As they expected, They were able to prepare long titanate nanotubes with lengths two orders longer than the short nanotube produced by the static hydrothermal method. | |
| Chen points out that this stirring hydrothermal method can be extended to other nanostructured systems, opening up new opportunities for manufacturing advanced functional materials for high-performance energy storage devices. | |
| Furthermore, for the first time the team reveals the relationship between nanotubular aspect ratio and electrochemical performance of LIBs. They show that the battery performance at high charging/discharging rate is dramatically boosted with increased aspect ratio, due to the optimization of electronic/ionic transport properties within the electrode materials. | |
| State-of-art TiO2 nanotubular electrode material produced by the traditional hydrothermal technique for high-rate battery applications requires additives – e.g., polymeric binders and conductive agent – due to their short length (up to one-micrometer) which decreases the energy and power density in terms of mass and volume. | |
| In contrast, the newly developed, elongated TiO2 nanotube result in an additive-free electrode system. | |
| As a proof of concept, the researchers used their TiO2 nanotubes to fabricate a robust three-dimensional nanotubular cross-linked network anode electrode. | |
| "Our ultrafast rechargeable lithium ion battery demonstrates high-rate charging-discharging (<3 min per charging, 8.4 A/g) for more than 10,000 cycles with a superior capacity (ca. 114 mAh g-1); this is equivalent to a product lifetime of more than 25 years, assuming one charging per day," Chen sums up the results. | |
| One of the challenges that the team is working on concerns the upscaling of this home built lab equipment. "We need to improve our design and explore experimental conditions – such as the stirring rate, temperature, and the loading concentration of the materials – for achieving large-scale synthesis with uniform morphology of the product," says Chen. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
