| Posted: Jan 05, 2015 | |
Single-pot electrolytic synthesis of hydrogen and carbon fuels |
|
| (Nanowerk Spotlight) The search for non-fossil based, low carbon footprint fuels includes several approaches to using solar energy. Among them, artificial photosynthesis – using solar energy to split water generating hydrogen and oxygen – is often considered a 'Holy Grail' of chemistry which can offer a clean and portable source of energy supply as durable as the sunlight. Although massive efforts have been made in this area, many researchers have faced different types of challenges reaching from fundamental sciences to engineering and most of these processes operate at much less than 10% solar efficiency. | |
| Back in 2002, Stuart Licht a professor of Chemistry, presented a solar electrochemical theory that the full spectrum of sunlight was sufficient to split water to hydrogen fuel at over 50% solar conversion efficiency. They named this theory STEP (the Solar Thermal Electrochemical Process) and demonstrated it, among others, for the direct removal of atmospheric carbon dioxide (New solar-powered process removes carbon dioxide from the air and stores it as solid carbon), the CO2-free production of iron (Reinventing iron production using clean renewable energy instead of coal) and calcium oxide (Solar-powered cement production without carbon dioxide emissions). | |
| STEP is a different type of solar conversion process that, rather than electricity, forms useful chemicals as the product, and uses the sun's thermal energy to heat and lower the energy of reactions, and the sun's visible energy to provide electrical current to drive the chemical reactions. | |
| "The general use of solar thermal energy to lower the potential of useful electrolyses can be applied to liquid, gas, or solid phase electrolyte cells," Licht explains to Nanowerk. "In general, we have found an energy advantage in applying STEP to liquid, molten electrolyte cells." (We have reported on this concept in two previous Nanowerk Spotlights: A new class of high-energy rechargeable batteries – molten air and Improved molten air battery operates at lower temperatures). | |
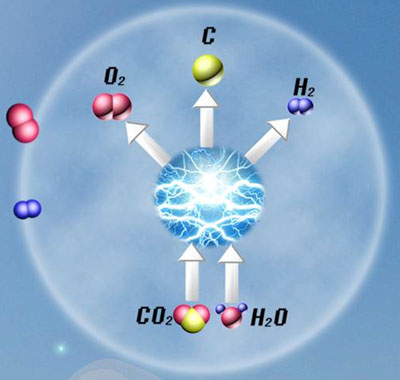
| Licht's group in the Department of Chemistry and Solar Institute at George Washington University has now successfully attempted to simultaneously co-generate hydrogen and solid carbon fuels from a mixed hydroxide/carbonate electrolyte in a 'single-pot' electrolytic synthesis at temperatures below 650°C. | |
 |
|
| Schematic of the process. (Image: Licht group, George Washington University) | |
| This is the first demonstration of the co-generation of hydrogen and carbon fuels at a single electrode and from a molten electrolyte. Here, fuel production can be driven entirely by solar energy using the STEP process in which solar thermal energy increases the system temperature to decrease electrolysis potentials. | |
| "The functionality to cogenerate hydrogen and carbon fuel at high current densities of several hundreds of mA/cm2, at low electrolysis potentials, and from water and carbon dioxide starting points, provides a significant step towards the development of renewable fuels," says Licht. | |
| The team has reported their findings in the December 23, 2014 online edition of Advanced Energy Materials ("A One-Pot Synthesis of Hydrogen and Carbon Fuels from Water and Carbon Dioxide"). | |
| The core advance of this work is the demonstration that both water and carbon dioxide can be absorbed and split in a single medium – a melted mixture of carbonate and hydroxide salts – providing a single chamber, which simultaneously splits water into hydrogen and carbon dioxide into hydrogen and carbon. | |
| STEP fuels (the solar thermal electrochemical process for fuels) uses inexpensive electrodes (nickel and steel) immersed in a molten electrolyte and can be heated by solar thermal and driven by solar photocurrent. | |
| A second advance in this study is the demonstration of a specialized barium/calcium salt that stabilizes the oxygen-generating nickel electrode. | |
| "STEP fuels are scaleable and we are in the process of scaling our electrolysis chamber from amps to hundreds of amps," notes Licht. "The demonstrated co-synthesis of hydrogen and solid carbon in this study, STEP organic ("STEP organic synthesis: an efficient solar, electrochemical process for the synthesis of benzoic acid"), as well as our previous synthesis of carbon monoxide fuel from carbon dioxide in these same molten salts suggests that the one-pot efficient synthesis of higher order fuels (hydrocarbons) are viable. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
