| Posted: Aug 25, 2010 | |
Reinventing iron production using clean renewable energy instead of coal |
|
| (Nanowerk Spotlight) Along with control of fire, iron smelting is one of the founding technological pillars of civilization. Industry has used the same basic process to make iron for over 3000 years. Yet, it is also one of the major global sources of greenhouse gas release. Iron, a basic commodity, is still produced by the greenhouse gas intensive reduction of iron oxide by carbon-coke and currently accounts for the release of one quarter of worldwide carbon dioxide emissions by industry. For instance, on average 1.9 tonnes of carbon dioxide are emitted for every tonne of steel produced. Due to a large share of coal in the energy mix of current production technology, the CO2 emissions are high. | |
| Through a new understanding of the chemistry of iron at high temperature, researchers have uncovered an effective new carbon-dioxide-free process to form iron. | |
| "We have shown a novel route to generate iron metal by the electrolysis of dissolved iron oxide salts in molten carbonate electrolytes," Stuart Licht, a professor in the Department of Chemistry and Solar Institute at George Washington University, explains to Nanowerk. "This process will prevent the extensive release of carbon dioxide, which currently accompanies the formation of iron metal from iron ores." | |
| These results come quite unexpected, due to the previously reported insolubility of iron oxide in carbonates, but Licht and his team have shown that their process can easily form pure metal iron from the two prevalent iron ores, hematite and magnetite. | |
| The unexpected solubility of iron oxides in lithium carbonate electrolytes, coupled with facile charge transfer and and a sharp decrease in iron electrolysis potentials with increasing temperature, provides a new route for iron production. | |
| "We have found that iron ore has a solubility product which is substantially larger, 1017 times (100,000,000,000,000,000x) larger than previously thought in high temperature carbonate liquids" says Licht. "This provides an ideal medium to form iron by our new STEP (Solar Thermal Electrochemical) energy conversion process." | |
| We have reported on this new STEP process in a recent Nanowerk Spotlight ("New solar-powered process removes carbon dioxide from the air and stores it as solid carbon"). | |
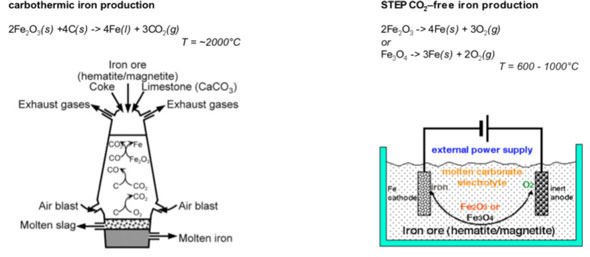 |
|
| Old and new iron production: Comparison of the industrial production of iron (left) and the STEP carbon-dioxide-free production of iron (right). The new process can utilize renewable energy power to drive iron formation, and doesn't release carbon dioxide because it doesn't burn coal. Alternatively, the new process can also be driven by fossil fuel electrical power, which as described in the study generates less CO2 than industry's current (carbothermic) process. (Image: Dr. Licht, George Washington University) | |
| Reporting his findings in a recent online issue of Chemical Communications ("High solubility pathway for the carbon dioxide free production of iron"), Licht has found a new way to use electrolysis – a process that uses electricity rather than chemicals to create a reaction – to covert iron ore to iron metal. | |
| This high temperature electrolysis requires little energy, and can be powered through conventional or renewable energy sources to reduce or completely eliminate carbon dioxide emissions. | |
| "When powered by STEP, the electrolysis process is carbon dioxide free, creating no global warming gas emissions when converting the ore into metal" says Licht. "By using both solar thermal energy and visible sunlight, the STEP process – diagrammed below – converts more solar energy than the best solar cells, as it uses excess solar heat (energy discarded by solar cells) to drive iron production." | |
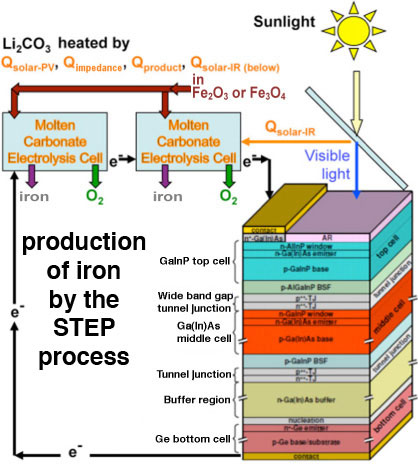 |
|
| Schematic depiction of the STEP process. (Image: Dr. Licht, George Washington University) | |
| In the STEP process, the iron is formed at unusually low energy, and without release of carbon dioxide gas. The extraction process called electrolysis occurs in an unusual molten lithium carbonate solution, in which electrons, rather than chemicals are used to convert the metal salts back to metal. The extracted iron is cleaned and contains pure iron metal. | |
 |
|
| Photographs of iron electrolysis product in molten carbonate from hematite (left side) or magnetite (right side). (Image: Dr. Licht, George Washington University) | |
| Licht notes that, in addition to iron production, the team has already successfully applied the STEP process to using solar energy to efficiently capture carbon (see our previous Spotlight) and hydrogen fuel from water, and are in the midst of applying the STEP process to a variety of useful energetic molecules including other metals and bleach at high solar efficiency from naturally occurring resources. | |
| He points out that the next challenge is looking for support to scale-up STEP iron production from the laboratory to the outdoor test environment. "A key part of the STEP process operates in similar (but reverse) manner to MCFCs (molten carbonate fuel cells), which have already faced, and solved, the materials issues of operating in these unusual high temperature molten carbonate conditions." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
