| Posted: Nov 24, 2016 | |
Improving the performance of lithium-sulfur batteries with coaxial nanotubes |
|
| (Nanowerk Spotlight) Lithium-sulfur (Li-S) batteries, which employ sulfur as cathode and metallic lithium as anode materials, have been extensively studied as promising alternatives to the widely used lithium-ion batteries because – theoretically – they can render 3-6 times higher energy density (2600 Wh kg-1) than conventional lithium-ion batteries with a theoretical value of 400-600 Wh kg-1. | |
| However, due to the intrinsic insulating nature of the active material (sulfur and lithium sulfide), Li–S batteries have suffered from low utilization of sulfur and thus low energy density. Fabricating electrodes with higher mass ratio of sulfur has therefore been a key focus of Li-S battery research. | |
| Moreover, during the charging and discharging process, the reaction intermediates (lithium polysulfides) are highly soluble in the ether-based electrolyte. The dissolved lithium polysulfides will shuttle to the anode side and react with it, causing undesired discharging (‘shuttle effect’). | |
| Therefore, Li–S battery is facing challenges of low Coulombic efficiency and poor cyclic stability. | |
| Over the past decade, much effort has been devoted to solving these problems. | |
| In new work, researchers at The University of Texas at Austin have designed PPy-MnO2 coaxial nanotubes with adjustable MnO2 content to encapsulate sulfur as a high-performance cathode for Li-S batteries. | |
| "Compared with pure PPy encapsulated sulfur, the S/PPy-MnO2 composites show much enhanced electrochemical performance, including Coulombic efficiency, cyclic stability, and rate capability," Guihua Yu, an Assistant Professor of Materials Science at the University of Texas at Austin, who led this work, explains to Nanowerk. "MnO2-PPy coaxial nanotubes provide a highly conductive matrix for sulfur and more importantly, strong trapping ability for polysulfides." | |
| As Yu's team reports in Nano Letters ("In Situ Reactive Synthesis of Polypyrrole-MnO2 Coaxial Nanotubes as Sulfur Hosts for High-Performance Lithium–Sulfur Battery"), the S/PPy-MnO2 composites show greatly improved electrochemical performance, including Coulombic efficiency, cyclic stability, and rate capability. | |
| The controlled deposition of discharging product (lithium sulfide) in S/PPy-MnO2 composites is also another key factor for cyclic stability. If lithium sulfide is randomly deposited on the surface of an electrode, the insulating nature will make it lose electric contact with the current collector and can not be used in the following recharging process. | |
| "We have achieved a stable Coulombic efficiency of ~98.6% and a decay rate of <0.07% per cycle with 500 cycles at 1C-rate (charge/discharge at 1 hour) for polypyrrole-MnO2 nanotubes encapsulated sulfur with 5 wt% of MnO2," Yu notes. | |
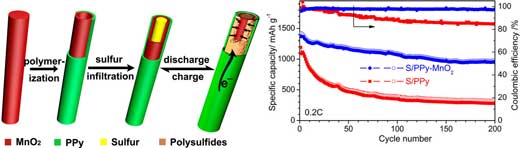 |
|
| Schematic illustration of polypyrrole-manganese dioxide (PPy-MnO2) coaxial nanotubes to accommodate sulfur for high-performance Li–S battery. Comparison of cyclic performance of S/PPy-MnO2 and S/PPy at 0.2C. (Reprinted with permission by American Chemical Society) (click on image to enlarge) | |
| The facile and controllable method to fabricate MnO2-conductive polymer nanotubes can be a useful strategy for the design and synthesis of scalable, high-performance Li–S batteries. | |
| "Li–S batteries with metallic lithium anodes face a safety issue because of the growth of lithium dendrites that can penetrate the separator and short-circuit the battery," Yu points out. "To avoid using a metallic lithium anode, lithium sulfide (Li2S) could be used as the cathode directly, which can be coupled with anodes such as graphite, silicon and tin to assemble a full battery with high energy density. We are now researching novel designs and synthesis methods of Li2S-based electrodes for high energy Li–S batteries." | |
| Researchers have found that several metal oxides such as MnO2 are effective materials to trap polysulfides and hence can greatly suppress the shuttle effect. However, the insulating nature of metal oxides cannot provide electron conductive pathways for sulfur. | |
| "PPy is also widely used as the conductive additive for electrodes, but fails to trap polysulfides," says Yu. "Simply mixing of the two materials is not favored because there is no space to accommodate sulfur. Fortunately, we found from the literature that MnO2 is an efficient initiator for the polymerization of PPy." | |
| The team therefore used MnO2 nanowires as both the initiator and template for PPy to form PPy-MnO2 coaxial nanotubes with enough internal space to encapsulate sulfur. | |
| "Our facile and controllable method to fabricate MnO2-conductive polymer nanotubes represents a useful strategy for the design and synthesis of scalable, high-performance Li-S batteries," concludes Yu. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
