| Posted: Dec 07, 2016 | |
Enhanced fuel cell performance using one atom thick 2D material |
|
| (Nanowerk Spotlight) Methanol fuel cells are widely considered as a potential source of future energy due to the usage of methanol as a liquid fuel; simplicity in operation; higher energy density of methanol fuel; high power density obtained etc. However their commercialization is greatly hindered by methanol crossover taking place in the membrane area of fuel cells, leading to short circuits and greatly affecting overall performance. | |
| More precisely, this problem could be defined as diffusion of methanol from anode to cathode through the membrane. This methanol – instead of reacting on the anode side – also reacts on the cathode side, thereby generating a short circuit. | |
| In previous research, different materials have been used and researchers have reported improved performance by reducing the methanol crossover. But all of these results showed a decrease in methanol crossover at the same time with reduced proton transport, which is one of dominant factors for fuel cell performance. | |
| By using two-dimensional (2D) materials – graphene and hexagonal boron nitride (hBn) – in methanol fuel cell systems, we now have overcome this bottleneck. | |
| Inspired by the Nobel Laureate Andre Geim's work on proton transport through single-layer graphene and other 2D materials (Nature, "Proton transport through one-atom-thick crystals"), we have reported for the first time, the testing of 2D materials in methanol fuel cells (Advanced Energy Materials, "2D Crystals Significantly Enhance the Performance of a Working Fuel Cell"). | |
 |
|
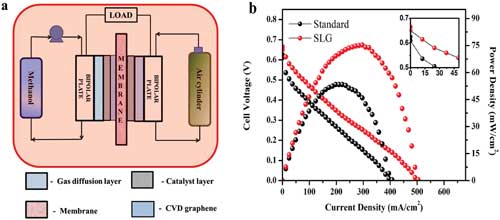
| a) Direct methanol fuel cell experimental set up and membrane electrode assembly (MEA) configuration. b) Polarization curve comparison for a standard and MEA with single layer graphene (SLG) at 70°C, 1 m methanol, 1 L min-1 of air. (Reprinted with permission by Wiley-VCH Verlag) (click on image to enlarge) | |
| In our paper, we show that the methanol fuel cell containing 2D materials has decreased methanol crossover by 40%, with no changes in proton conductivity observed, leading to performance improvements of up to 50%. | |
| In conclusion, we demonstrate that the addition of the graphene layer has significantly changed the properties of the membrane electrode assembly by reducing the methanol permeability with no measurable difference in proton conductivity at elevated temperatures. | |
| Since the membrane occupies most volume of a fuel cell, and the 2D graphene and hBN materials are only approx. 1 Ångström thick (10-10 metres), this could lead to miniaturized fuel cells. | |
| However, achieving this as obtaining coherent sheet of single-layer graphene on a larger electrode area suitable for use in fuel cell still remains a challenge and requires more work. | |
| Generally, though, the methanol inhibition and proton transport phenomenon of these 2D materials could also be utilized in other fuel cell types. | |
| Based on the results obtained, our research team received a £0.5 million 'Adventurers in Energy' grant to pursue further work on these 2D materials. | |
|
By Prabhuraj Balakrishnan, PGR student in the School of Chemical Engineering and Analytical Science, University of Manchester
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
