| Posted: Jul 11, 2008 | |
Nanotechnology straws - capillary action at the nanoscale |
|
| (Nanowerk Spotlight) Various techniques are being developed to enhance the already impressive properties of carbon nanotubes (CNTs) further by combining them with other materials. We have covered plenty of examples in our Spotlights. For instance, encapsulating carbon nanofibers with CNTs transforms cheap commercial carbon nanotubes into highly efficient carbon for electrochemical energy storage applications (Converting conventional nanotubes into superior carbon for batteries). Another study demonstrated that the redox properties of iron and iron oxide particles are tunable via encapsulation within CNTs, suggesting that a host-guest interaction between the confined metal particles and CNTs, which is different from that on the outside of the nanotubes (see: Ethanol production inside carbon nanotubes). For a more general example read Nanochemistry inside carbon nanotubes. | |
| Researchers are still busy trying to understand some of the CNT basics, for instance something as fundamental as "how do nanotubes grow"? How can their various properties – electronic, transport, or mechanical – be modified? Or how can you make use of CNT's structure and properties to build novel nanotools (see our recent Spotlight: Nanotechnology pipettes as tools to demystify and modify biological processes). | |
| A new model demonstrates that sufficiently small liquid metal droplets can be drawn inside a CNT via capillary action. This could have interesting implications for scientists' understanding of the growth of CNTs and may lead to new synthetic routes for composite nanofibers as well as production of nanodevices from CNTs and metal nanoparticles. | |
| "We have shown that capillary forces can result in the absorption of molten metal droplets by carbon nanotubes" Dr. Shaun Hendy tells Nanowerk. "Capillary forces are one of the effects of surface tension and they can cause liquids to be drawn up by small tubes, including nanotubes. However, if the liquid is molten metal, and the nanotube is carbon, this was not expected to occur. Liquid metals generally prefer to minimize their contact area with carbon – for instance a droplet of molten iron will remain almost spherical when placed on a graphite sheet or piece of diamond. Because of this, it was expected that capillary forces would not be strong enough to draw metal droplets into the carbon tubes. However, we have shown that when the metal droplet is sufficiently small, the effects of surface tension do in fact become large enough to cause capillary draw up by a carbon nanotube." | |
| Hendy, a Senior Scientist at Industrial Research Ltd and the MacDiarmid Institute for Advanced Materials and Nanotechnology, together with his PhD student Dmitri Schebarchov from Victoria University of Wellington's School of Chemical and Physical Sciences in New Zealand, report their findings in the July 3, 2008 online edition of Nano Letters ("Capillary Absorption of Metal Nanodroplets by Single-Wall Carbon Nanotubes"). | |
| Hendy notes that their model demonstrates an important phenomenon because nanoscale metal droplets are frequently used as catalysts to grow CNTs. "By providing a theoretical description of this capillary absorption phenomenon and confirming it with computer simulations, we are able to explain why metal particles are frequently observed within carbon nanotubes after growth," he says. "The result also has implications for understanding the growth of carbon nanotubes from metal catalysts. For instance, if the catalyst droplet is too small relative to the growing tube, it will become encapsulated by the tube and growth will cease." | |
| The observation that very small droplets of a liquid could be drawn up by capillary tubes when larger droplets of the same liquid could not, was made by Abraham Marmur from the Technion in Israel in the 1980s ("Penetration of a small drop into a capillary"). | |
| Hendy says that, in essence, he and Schebarchov have rediscovered his effect in the metal droplet-carbon nanotube system, and confirmed that the phenomena occur in this system using computer simulation. | |
| The two scientists stumbled across these findings by accident. "We were studying the melting of metal nanoparticles on graphite using computer simulation" they explain. "As carbon nanotubes are simply rolled up graphite sheets, we thought it would be interesting to try putting the particles inside carbon nanotubes to look at the effect on melting. The question then arose as to how we should put a particle in a tube before we started the simulation. In a computer simulation you can arrange the atoms however you want of course, but we thought it would be more interesting to try putting the metal atoms in using capillary absorption – luckily it worked!" | |
 |
|
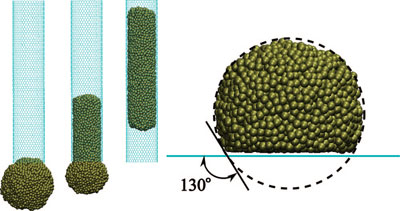
| (left) Snapshots of the molten palladium (Pd) particle (46 Å in diameter) being absorbed by a 30 Å diameter single-wall CNT for ε = 31.8 meV. (right) Snapshot of the molten Pd particle on a graphene sheet. For this value of ε the contact angle formed by the droplet on a flat sheet of graphene is very close to 130°. Note that the meniscus formed by the encapsulated droplet is clearly convex. (Reprinted with permission from American Chemical Society) | |
| In their model, the two scientists simply compare the total surface free energies of three scenarios: a spherical droplet near the opening of a tube; a droplet of the same volume partially absorbed inside the tube; and a droplet of the same volume fully encapsulated inside the tube (they neglect the effects of line tension and gravity and assume that the density remains constant upon encapsulation). | |
| "As expected," says Hendy "our model predicts that liquid droplets exhibiting at least partial wetting of the tube will undergo spontaneous capillary action and fill the tube, regardless of droplet size. This is consistent with the macroscopic theory of capillarity. However, according to the model, even if the contact angle is greater than 90°, droplets of nonwetting melts will prefer encapsulation provided their initial radius of curvature is small enough." | |
| According to the scientists, a simple physical explanation for why nonwetting droplets may undergo spontaneous capillary action lies in the consideration of the Laplace pressure – which describes the difference in pressure between the inside and outside of a droplet due to surface tension – acting on the protruding spherical cap. This extra force acts toward the tube and drives the droplet inside the hollow, even if the pressure associated with the meniscus at the other end opposes capillary action. | |
| "We think our model goes some way to explaining why catalyst particles frequently end up absorbed in carbon nanotubes after growth" Hendy says. "We think it also partly explains the relationship that has been observed between catalyst particle size and the final diameter of the carbon nanotubes that grow from the particle." | |
| There is still only a rather sketchy understanding of carbon nanotube growth, and this limits researchers' ability to grow carbon nanotubes of a desired diameter and chirality (the chirality of a tube is related to the way the graphite sheet is wrapped up and determines the electronic properties of the final tube). | |
| The diameter and chirality are probably determined early during the growth process when a graphite cap nucleates on the metal droplet surface – capillary forces ought to play a role as the cap lifts off the droplet surface to form a tube. Hendy points out that he and his team would like to understand this process better and develop a simple model to help predict the final properties of the carbon nanotube from the properties of the catalyst particle used. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
