Showing Spotlights 177 - 184 of 316 in category All (newest first):
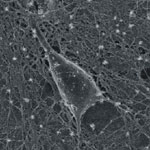 Neural engineering is an emerging discipline that uses engineering techniques to investigate the function and manipulate the behavior of the central or peripheral nervous systems. Neural engineering is highly interdisciplinary and relies on expertise from computational neuroscience, experimental neuroscience, clinical neurology, electrical engineering and signal processing of living neural tissue, and encompasses elements from robotics, computer engineering, neural tissue engineering, materials science, and nanotechnology. In order for neural prostheses to augment or restore damaged or lost functions of the nervous system they need to be able to perform two main functions: stimulate the nervous system and record its activity. To do that, neural engineers have to gain a full understanding of the fundamental mechanisms and subtleties of cell-to-cell signaling via synaptic transmission, and then develop the technologies to replicate these mechanisms with artificial devices and interface them to the neural system at the cellular level. A group of European researchers has now shown that carbon nanotubes may become the ideal material for repairing damaged brain tissue.
Neural engineering is an emerging discipline that uses engineering techniques to investigate the function and manipulate the behavior of the central or peripheral nervous systems. Neural engineering is highly interdisciplinary and relies on expertise from computational neuroscience, experimental neuroscience, clinical neurology, electrical engineering and signal processing of living neural tissue, and encompasses elements from robotics, computer engineering, neural tissue engineering, materials science, and nanotechnology. In order for neural prostheses to augment or restore damaged or lost functions of the nervous system they need to be able to perform two main functions: stimulate the nervous system and record its activity. To do that, neural engineers have to gain a full understanding of the fundamental mechanisms and subtleties of cell-to-cell signaling via synaptic transmission, and then develop the technologies to replicate these mechanisms with artificial devices and interface them to the neural system at the cellular level. A group of European researchers has now shown that carbon nanotubes may become the ideal material for repairing damaged brain tissue.
Dec 29th, 2008
 Researchers have demonstrated that salmon DNA can be used to develop a simple and scalable method for sorting carbon nanotubes that reduces the cost, as compared to commonly used synthetic DNA, by a factor of 1,000. Before carbon nanotubes (CNTs), especially single-walled ones, can live up to the many expectations for their use in nanoelectronics, researchers have to overcome a seemingly trivial but nonetheless major obstacle: how to separate a produced batch of nanotubes according to their properties such as diameter, length, chirality and electronic attributes. Current production methods for CNTs result in a jumble of units with different properties, all lumped together in bundles, and often blended with some amount of amorphous carbon. These mixtures are of little practical use since many advanced applications, especially for nanoelectronics, are sensitively dependent on tube structures and the slightest deviation from a desired set of parameters can lead to vastly different performance results.
Researchers have demonstrated that salmon DNA can be used to develop a simple and scalable method for sorting carbon nanotubes that reduces the cost, as compared to commonly used synthetic DNA, by a factor of 1,000. Before carbon nanotubes (CNTs), especially single-walled ones, can live up to the many expectations for their use in nanoelectronics, researchers have to overcome a seemingly trivial but nonetheless major obstacle: how to separate a produced batch of nanotubes according to their properties such as diameter, length, chirality and electronic attributes. Current production methods for CNTs result in a jumble of units with different properties, all lumped together in bundles, and often blended with some amount of amorphous carbon. These mixtures are of little practical use since many advanced applications, especially for nanoelectronics, are sensitively dependent on tube structures and the slightest deviation from a desired set of parameters can lead to vastly different performance results.
Dec 16th, 2008
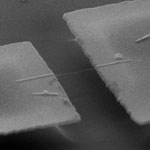 Shrinking device size to nanometer dimensions presents many fascinating opportunities such as manipulating nano objects with nanotools, measuring mass in attogram ranges, sensing forces at femtonewton scales, and inducing gigahertz motion, among other new possibilities waiting to be discovered. The two principal components common to most electromechanical systems irrespective of scale are a mechanical element and transducers. The mechanical element either deflects or vibrates in response to an applied force. Depending on their type, the mechanical elements can be used to sense static or time-varying forces. The transducers in microelectromechanical systems (MEMS) and nanoelectromechanical systems (NEMS) convert mechanical energy into electrical or optical signals and vice versa. A Spanish team has now demonstrated an ultrasensitive carbon nanotube based mass sensor in which they measured chromium atoms with a mass resolution of only 1.4 zeptograms.
Shrinking device size to nanometer dimensions presents many fascinating opportunities such as manipulating nano objects with nanotools, measuring mass in attogram ranges, sensing forces at femtonewton scales, and inducing gigahertz motion, among other new possibilities waiting to be discovered. The two principal components common to most electromechanical systems irrespective of scale are a mechanical element and transducers. The mechanical element either deflects or vibrates in response to an applied force. Depending on their type, the mechanical elements can be used to sense static or time-varying forces. The transducers in microelectromechanical systems (MEMS) and nanoelectromechanical systems (NEMS) convert mechanical energy into electrical or optical signals and vice versa. A Spanish team has now demonstrated an ultrasensitive carbon nanotube based mass sensor in which they measured chromium atoms with a mass resolution of only 1.4 zeptograms.
Nov 18th, 2008
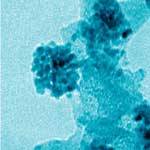 Adding yet another twist to the emerging debate about the potential risks of nanomaterials, researchers have demonstrated how difficult it is to map out the health effects of nanoparticles. They have shown that, even if a certain nanoparticle does not appear toxic by itself, the interaction between this nanoparticle and other common compounds in the human body may cause serious problems to cell functions. On one hand, this effect could be used to great advantage in nanomedicine for killing cancer cells. On the other hand, unfortunately, it is unknown at present whether the same effect could be observed with healthy cells as well. Since the number of possible combinations of nanoparticles and various biomolecules is immense, it is practically impossible to research them systematically. This latest example of the risk-benefit dichotomy of nanotechnology just shows how thin the line is between promising applications such as effective cancer killers and the unknown risks posed by unintentional effects of exactly the same applications.
Adding yet another twist to the emerging debate about the potential risks of nanomaterials, researchers have demonstrated how difficult it is to map out the health effects of nanoparticles. They have shown that, even if a certain nanoparticle does not appear toxic by itself, the interaction between this nanoparticle and other common compounds in the human body may cause serious problems to cell functions. On one hand, this effect could be used to great advantage in nanomedicine for killing cancer cells. On the other hand, unfortunately, it is unknown at present whether the same effect could be observed with healthy cells as well. Since the number of possible combinations of nanoparticles and various biomolecules is immense, it is practically impossible to research them systematically. This latest example of the risk-benefit dichotomy of nanotechnology just shows how thin the line is between promising applications such as effective cancer killers and the unknown risks posed by unintentional effects of exactly the same applications.
Nov 17th, 2008
 If current research is an indicator, wearable electronics will go far beyond just very small electronic devices or wearable, flexible computers. Not only will these devices be embedded in textile substrates but an electronics device or system could ultimately become the fabric itself. Electronic textiles (e-textiles) will allow the design and production of a new generation of garments with distributed sensors and electronic functions. Such e-textiles will have the revolutionary ability to sense, act, store, emit, and move - think biomedical monitoring functions or new man-machine interfaces - while ideally leveraging an existing low-cost textile manufacturing infrastructure. A recent research report proposes to make conductive, carbon nanotube-modified cotton yarn. This would offer a uniquely simple yet remarkably functional solution for smart textiles - close in feel and handling to normal fabric - yet with many parameters exceeding existing solutions.
If current research is an indicator, wearable electronics will go far beyond just very small electronic devices or wearable, flexible computers. Not only will these devices be embedded in textile substrates but an electronics device or system could ultimately become the fabric itself. Electronic textiles (e-textiles) will allow the design and production of a new generation of garments with distributed sensors and electronic functions. Such e-textiles will have the revolutionary ability to sense, act, store, emit, and move - think biomedical monitoring functions or new man-machine interfaces - while ideally leveraging an existing low-cost textile manufacturing infrastructure. A recent research report proposes to make conductive, carbon nanotube-modified cotton yarn. This would offer a uniquely simple yet remarkably functional solution for smart textiles - close in feel and handling to normal fabric - yet with many parameters exceeding existing solutions.
Nov 14th, 2008
 As we have show before, nanotechnology applications could provide decisive technological breakthroughs in the energy sector and have a considerable impact on creating the sustainable energy supply that is required to make the transition from fossil fuels. Although we love to write about all the clean and green applications that will be nanotechnology enabled, the harsh reality is that dirty energy is still fuelling our way of life. No matter if you are a member of the "drill, baby, drill" crowd or if you are actively involved in saving energy and think that the development of renewable energies can't come fast enough, we have to live with the fact that the world's energy production will continue to depend on oil, gas and coal for quite a few more years. But even here, nanotechnology applications might offer some improvements. A new report shows that nanotechnology, in the form of carbon nanotube rubber composites, could help to significantly enhance oil production efficiency by allowing to probe and drill deeper wells.
As we have show before, nanotechnology applications could provide decisive technological breakthroughs in the energy sector and have a considerable impact on creating the sustainable energy supply that is required to make the transition from fossil fuels. Although we love to write about all the clean and green applications that will be nanotechnology enabled, the harsh reality is that dirty energy is still fuelling our way of life. No matter if you are a member of the "drill, baby, drill" crowd or if you are actively involved in saving energy and think that the development of renewable energies can't come fast enough, we have to live with the fact that the world's energy production will continue to depend on oil, gas and coal for quite a few more years. But even here, nanotechnology applications might offer some improvements. A new report shows that nanotechnology, in the form of carbon nanotube rubber composites, could help to significantly enhance oil production efficiency by allowing to probe and drill deeper wells.
Nov 11th, 2008
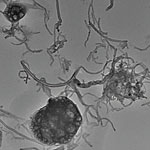 The toxicity issues surrounding carbon nanotubes (CNTs) are highly relevant for two reasons: Firstly, as more and more products containing CNTs come to market, there is a chance that free CNTs get released during their life cycles, most likely during production or disposal, and find their way through the environment into the body. Secondly, and much more pertinent with regard to potential health risks, is the use of CNTs in biological and medical settings. CNTs interesting structural, chemical, electrical, and optical properties are explored by numerous nanomedicine research groups around the world with the goal of drastically improving performance and efficacy of biological detection, imaging, and therapy applications. In many of these envisaged applications, CNTs would be deliberately injected or implanted in the body. While it has been shown that carbon nanotubes can indeed act as a means for drug delivery, negative effects such as unusual and robust inflammatory response, oxidative stress and formation of free radicals, and the accumulation of peroxidative products have also been found as a result of carbon nanotubes and their accumulated aggregates. As a possible solution, scientists have provided compelling evidence of the biodegradation of carbon nanotubes by horseradish peroxidase and hydrogen peroxide over the period of several weeks. This marks a promising possibility for nanotubes to be degraded by horseradish peroxidase in environmentally relevant settings.
The toxicity issues surrounding carbon nanotubes (CNTs) are highly relevant for two reasons: Firstly, as more and more products containing CNTs come to market, there is a chance that free CNTs get released during their life cycles, most likely during production or disposal, and find their way through the environment into the body. Secondly, and much more pertinent with regard to potential health risks, is the use of CNTs in biological and medical settings. CNTs interesting structural, chemical, electrical, and optical properties are explored by numerous nanomedicine research groups around the world with the goal of drastically improving performance and efficacy of biological detection, imaging, and therapy applications. In many of these envisaged applications, CNTs would be deliberately injected or implanted in the body. While it has been shown that carbon nanotubes can indeed act as a means for drug delivery, negative effects such as unusual and robust inflammatory response, oxidative stress and formation of free radicals, and the accumulation of peroxidative products have also been found as a result of carbon nanotubes and their accumulated aggregates. As a possible solution, scientists have provided compelling evidence of the biodegradation of carbon nanotubes by horseradish peroxidase and hydrogen peroxide over the period of several weeks. This marks a promising possibility for nanotubes to be degraded by horseradish peroxidase in environmentally relevant settings.
Nov 10th, 2008
 Forget boxy loudspeakers. Researchers have now found that just a piece of carbon nanotube thin film could be a practical magnet-free loudspeaker simply by applying an audio frequency current through it. These loudspeakers - which are only tens of nanometers thick, transparent, flexible, and stretchable - can be tailored into many shapes and mounted on a variety of insulating surfaces, such as room walls, ceilings, pillars, windows, flags, and clothes without area limitations. The scientists demonstrated that their CNT loudspeakers can generate sound with wide frequency range, high sound pressure level, and low total harmonic distortion. Another advantage compared to conventional loudspeakers is that the CNT loudspeakers don't vibrate and are damage tolerant. They will work even if part of the thin film is torn or damaged.
Forget boxy loudspeakers. Researchers have now found that just a piece of carbon nanotube thin film could be a practical magnet-free loudspeaker simply by applying an audio frequency current through it. These loudspeakers - which are only tens of nanometers thick, transparent, flexible, and stretchable - can be tailored into many shapes and mounted on a variety of insulating surfaces, such as room walls, ceilings, pillars, windows, flags, and clothes without area limitations. The scientists demonstrated that their CNT loudspeakers can generate sound with wide frequency range, high sound pressure level, and low total harmonic distortion. Another advantage compared to conventional loudspeakers is that the CNT loudspeakers don't vibrate and are damage tolerant. They will work even if part of the thin film is torn or damaged.
Nov 3rd, 2008
 Neural engineering is an emerging discipline that uses engineering techniques to investigate the function and manipulate the behavior of the central or peripheral nervous systems. Neural engineering is highly interdisciplinary and relies on expertise from computational neuroscience, experimental neuroscience, clinical neurology, electrical engineering and signal processing of living neural tissue, and encompasses elements from robotics, computer engineering, neural tissue engineering, materials science, and nanotechnology. In order for neural prostheses to augment or restore damaged or lost functions of the nervous system they need to be able to perform two main functions: stimulate the nervous system and record its activity. To do that, neural engineers have to gain a full understanding of the fundamental mechanisms and subtleties of cell-to-cell signaling via synaptic transmission, and then develop the technologies to replicate these mechanisms with artificial devices and interface them to the neural system at the cellular level. A group of European researchers has now shown that carbon nanotubes may become the ideal material for repairing damaged brain tissue.
Neural engineering is an emerging discipline that uses engineering techniques to investigate the function and manipulate the behavior of the central or peripheral nervous systems. Neural engineering is highly interdisciplinary and relies on expertise from computational neuroscience, experimental neuroscience, clinical neurology, electrical engineering and signal processing of living neural tissue, and encompasses elements from robotics, computer engineering, neural tissue engineering, materials science, and nanotechnology. In order for neural prostheses to augment or restore damaged or lost functions of the nervous system they need to be able to perform two main functions: stimulate the nervous system and record its activity. To do that, neural engineers have to gain a full understanding of the fundamental mechanisms and subtleties of cell-to-cell signaling via synaptic transmission, and then develop the technologies to replicate these mechanisms with artificial devices and interface them to the neural system at the cellular level. A group of European researchers has now shown that carbon nanotubes may become the ideal material for repairing damaged brain tissue. 
 Subscribe to our Nanotechnology Spotlight feed
Subscribe to our Nanotechnology Spotlight feed





