Showing Spotlights 481 - 488 of 627 in category All (newest first):
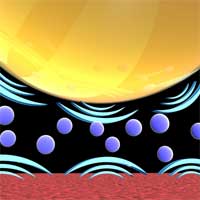 When two surfaces approach each other in air, they attract. A phenomenon that is explained by van der Waals forces that affect molecules' interaction. Without these - very weak - intermolecular forces, life as we know it would be impossible. They are responsible for a number of properties of molecular compounds, including crystal structures, condensing, melting and boiling points, surface tension, and densities. Intermolecular forces form molecules like enzymes, proteins, and DNA into the shapes required for biological activity. Van der Waals forces can also be repulsive, for instance when two surfaces approach each other in liquid: the same force which causes attraction in air (and which is responsible for so called stiction and adhesion) can be made repulsive by choosing the right combination of surface materials and intervening liquid. This force has the characteristic that it increases very rapidly with very small changes in separation when the surfaces are close to each other. Researchers now have shown that if repulsive van der Waals forces exist between two surfaces prior to their contact then friction is essentially precluded and supersliding is achieved. This opens the possibility that, in certain material systems, the controlled use of repulsive van der Waals forces could be a way to reduce, if not eliminate, friction.
When two surfaces approach each other in air, they attract. A phenomenon that is explained by van der Waals forces that affect molecules' interaction. Without these - very weak - intermolecular forces, life as we know it would be impossible. They are responsible for a number of properties of molecular compounds, including crystal structures, condensing, melting and boiling points, surface tension, and densities. Intermolecular forces form molecules like enzymes, proteins, and DNA into the shapes required for biological activity. Van der Waals forces can also be repulsive, for instance when two surfaces approach each other in liquid: the same force which causes attraction in air (and which is responsible for so called stiction and adhesion) can be made repulsive by choosing the right combination of surface materials and intervening liquid. This force has the characteristic that it increases very rapidly with very small changes in separation when the surfaces are close to each other. Researchers now have shown that if repulsive van der Waals forces exist between two surfaces prior to their contact then friction is essentially precluded and supersliding is achieved. This opens the possibility that, in certain material systems, the controlled use of repulsive van der Waals forces could be a way to reduce, if not eliminate, friction.
Feb 28th, 2008
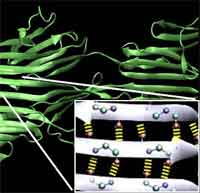 Much has been written about the fascinating properties of spider silk, a biopolymer that is stronger than steel and more elastic than rubber. The silken threads possess a unique combination of mechanical properties: strength, extensibility and toughness. Of course this begs the obvious question: How is it possible that spider silk, produced by little creatures that evolved about 400 million years ago, can be as strong as steel - a modern alloy that plays a critical role in our infrastructure and which still attracts considerable R&D investments in its production technology? What is perplexing is that the atomic interactions (H-bonds) in spider silk are actually 100 to 1,000 times weaker than those in steel, or than those in the superfiber Kevlar, where covalent bonds are used. Hydrogen bonds are the basic chemical bonds that hold together proteins, similar to trusses and beams in buildings, and play a key role in controlling the behavior of these structures. In order to reach silk's mechanical properties, most synthetic materials must be much denser, thus much heavier and consume much more energy during their synthesis and transport. New analysis performed at MIT's Laboratory for Atomistic and Molecular Mechanics shows that the intriguing strength of spider silk may be made possible by precisely controlling the number and the geometry of H-bonds at a characteristic length scale. The physical concept is that by making many small elements work together cooperatively, the weaknesses of the individual components can be overcome. All this must happen at the nanoscale in order to be effective.
Much has been written about the fascinating properties of spider silk, a biopolymer that is stronger than steel and more elastic than rubber. The silken threads possess a unique combination of mechanical properties: strength, extensibility and toughness. Of course this begs the obvious question: How is it possible that spider silk, produced by little creatures that evolved about 400 million years ago, can be as strong as steel - a modern alloy that plays a critical role in our infrastructure and which still attracts considerable R&D investments in its production technology? What is perplexing is that the atomic interactions (H-bonds) in spider silk are actually 100 to 1,000 times weaker than those in steel, or than those in the superfiber Kevlar, where covalent bonds are used. Hydrogen bonds are the basic chemical bonds that hold together proteins, similar to trusses and beams in buildings, and play a key role in controlling the behavior of these structures. In order to reach silk's mechanical properties, most synthetic materials must be much denser, thus much heavier and consume much more energy during their synthesis and transport. New analysis performed at MIT's Laboratory for Atomistic and Molecular Mechanics shows that the intriguing strength of spider silk may be made possible by precisely controlling the number and the geometry of H-bonds at a characteristic length scale. The physical concept is that by making many small elements work together cooperatively, the weaknesses of the individual components can be overcome. All this must happen at the nanoscale in order to be effective.
Feb 14th, 2008
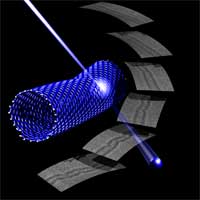 Electron microscopy is a delicate balancing act between using the highest energy electrons possible in order to obtain the best image while avoiding destruction of the sample under investigation. Trouble is, the electron beam does not just observe, it also interacts with the structure that is observed. This can be critical for fragile samples such as nanostructures: the transfer of energy from the electron beam can be sufficient that atoms are knocked right out of the material. In the worst case the sample is destroyed in front of your eyes. Scientists in Orsay, France have turned this problem to their advantage. They have developed a new approach to shaping nanomaterials atom by atom, using a scanning transmission electron microscope (STEM) as a nanometrically precise cutting tool. In this way they 'carve' their materials, such as carbon nanotubes, by removing individual atoms from specifically chosen locations. This technique has the added advantage that scientists can observe what they are doing at the same time. The new technique demonstrates a 'nanoelectron- lithography' of single walled nanotubes, a top down approach to locally control their nanostructures.
Electron microscopy is a delicate balancing act between using the highest energy electrons possible in order to obtain the best image while avoiding destruction of the sample under investigation. Trouble is, the electron beam does not just observe, it also interacts with the structure that is observed. This can be critical for fragile samples such as nanostructures: the transfer of energy from the electron beam can be sufficient that atoms are knocked right out of the material. In the worst case the sample is destroyed in front of your eyes. Scientists in Orsay, France have turned this problem to their advantage. They have developed a new approach to shaping nanomaterials atom by atom, using a scanning transmission electron microscope (STEM) as a nanometrically precise cutting tool. In this way they 'carve' their materials, such as carbon nanotubes, by removing individual atoms from specifically chosen locations. This technique has the added advantage that scientists can observe what they are doing at the same time. The new technique demonstrates a 'nanoelectron- lithography' of single walled nanotubes, a top down approach to locally control their nanostructures.
Feb 8th, 2008
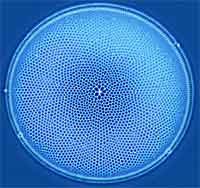 Diatoms are a major group of hard-shelled algae and one of the most common types of phytoplankton. A characteristic feature of diatom cells is that they are encased within a unique cell wall made of silica. Silicate materials are very important in nature and they are closely related to the evolution of living organisms. Diatom walls show a wide diversity in form, some quite beautiful and ornate, but usually consist of two symmetrical sides with a split between them, hence the group name. There is great potential for the use of diatoms in nanotechnology. This potential lies in the pores and channels which give rise to a greatly increased surface area, and the silica structure which lends itself to chemical modification. In addition there is a huge variety in the sizes and shapes of diatoms available, providing scope for the selection of a particular species of diatom tailored to a particular requirement. Researchers in the UK have demonstrated that the silica walls of diatoms can be used for the attachment of active biomolecules, such as antibodies, using either primary amine groups or the carbohydrate moiety. These modified structures can, therefore, be used for antibody arrays or for use in techniques such as immunoprecipitation.
Diatoms are a major group of hard-shelled algae and one of the most common types of phytoplankton. A characteristic feature of diatom cells is that they are encased within a unique cell wall made of silica. Silicate materials are very important in nature and they are closely related to the evolution of living organisms. Diatom walls show a wide diversity in form, some quite beautiful and ornate, but usually consist of two symmetrical sides with a split between them, hence the group name. There is great potential for the use of diatoms in nanotechnology. This potential lies in the pores and channels which give rise to a greatly increased surface area, and the silica structure which lends itself to chemical modification. In addition there is a huge variety in the sizes and shapes of diatoms available, providing scope for the selection of a particular species of diatom tailored to a particular requirement. Researchers in the UK have demonstrated that the silica walls of diatoms can be used for the attachment of active biomolecules, such as antibodies, using either primary amine groups or the carbohydrate moiety. These modified structures can, therefore, be used for antibody arrays or for use in techniques such as immunoprecipitation.
Feb 7th, 2008
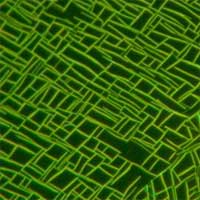 The vision of revolutionary bottom-up nanotechnology is based on a concept of molecular assembly technologies where nanoscale materials and structures self-assemble to microscale structures and finally to macroscopic devices and products. We are a long way from realizing this vision but researchers are busily laying the foundation for nanoscale engineering. Assembling nanoscopic components into macroscopic materials is an appealing goal but one of the enormous difficulties lies in bridging approximately six orders of magnitude that separate the nanoscale from the macroscopic world. Until machinery capable of automated and industrial-scale nano-assembly can be built, the parallelism of chemical synthesis and self-assembly is necessary when controlling materials at the nanoscale. An obvious direct approach to molecular nanotechnology therefore is to start with organic molecules as building blocks. Modest from the viewpoint of molecular manufacturing visionaries, but quite fascinating to a lot of scientists, research into nanofibers, as a modification of organic crystals, is making good progress. New research results coming out of Denmark offer the basis for a novel organic-molecule-based nanotechnological concept that allows for a multitude of applications in fundamental research and in device applications. Essentially, this concept is based on three steps: 1) directed self-assembled surface growth of nanofibers from functionalized molecules; 2) transfer and manipulation of individual fibers as well as of ordered arrays; and 3) device integration.
The vision of revolutionary bottom-up nanotechnology is based on a concept of molecular assembly technologies where nanoscale materials and structures self-assemble to microscale structures and finally to macroscopic devices and products. We are a long way from realizing this vision but researchers are busily laying the foundation for nanoscale engineering. Assembling nanoscopic components into macroscopic materials is an appealing goal but one of the enormous difficulties lies in bridging approximately six orders of magnitude that separate the nanoscale from the macroscopic world. Until machinery capable of automated and industrial-scale nano-assembly can be built, the parallelism of chemical synthesis and self-assembly is necessary when controlling materials at the nanoscale. An obvious direct approach to molecular nanotechnology therefore is to start with organic molecules as building blocks. Modest from the viewpoint of molecular manufacturing visionaries, but quite fascinating to a lot of scientists, research into nanofibers, as a modification of organic crystals, is making good progress. New research results coming out of Denmark offer the basis for a novel organic-molecule-based nanotechnological concept that allows for a multitude of applications in fundamental research and in device applications. Essentially, this concept is based on three steps: 1) directed self-assembled surface growth of nanofibers from functionalized molecules; 2) transfer and manipulation of individual fibers as well as of ordered arrays; and 3) device integration.
Feb 1st, 2008
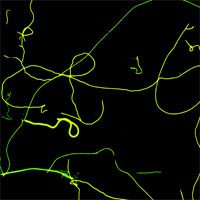 'Smart' is the key buzz word used by materials engineers when they describe the future of coatings, textiles, building structures, vehicles and just any material that you can think of. Materials are made 'smart' when they are engineered to have properties that change in a controlled manner under the influence of external stimuli such as mechanical stress, temperature, humidity, electric charge, magnetic fields etc. Nature of course is full with 'smart' materials that are capable of adapting to new tasks, are self-healing, and can self-assemble autonomously simply out of a solution of building blocks. Duplicating this feat with man-made materials will one day become a reality thanks to nanotechnology. Scientists not only dream about self-repairing cars or building walls that turn transparent like windows, they are actively working on the first steps towards these goals. Simple smart materials (that are not nanotechnology based) are already a reality, such as piezoelectric materials and shape memory alloys. Emerging nanotechnologies are now about to give scientists the tools to take smart materials to the next performance level. For instance, the European project Inteltex is developing a new, multifunctional textile that could be used as a wallpaper to detect temperature changes or chemical leakage or that could be used in medical and protective wear to monitor body temperature and mechanical stress. MIT's Institute for Soldier Nanotechnologies works on smart surfaces that switch properties. Nanotechnology-enabled smart materials are still very early days but basic progress is being made. Another small building block towards smart materials was recently reported by Italian researchers who demonstrated photo-switchable nanofibers based on the reversible transformation between two molecular photochemical states, exhibiting different chemico-physical characteristics.
'Smart' is the key buzz word used by materials engineers when they describe the future of coatings, textiles, building structures, vehicles and just any material that you can think of. Materials are made 'smart' when they are engineered to have properties that change in a controlled manner under the influence of external stimuli such as mechanical stress, temperature, humidity, electric charge, magnetic fields etc. Nature of course is full with 'smart' materials that are capable of adapting to new tasks, are self-healing, and can self-assemble autonomously simply out of a solution of building blocks. Duplicating this feat with man-made materials will one day become a reality thanks to nanotechnology. Scientists not only dream about self-repairing cars or building walls that turn transparent like windows, they are actively working on the first steps towards these goals. Simple smart materials (that are not nanotechnology based) are already a reality, such as piezoelectric materials and shape memory alloys. Emerging nanotechnologies are now about to give scientists the tools to take smart materials to the next performance level. For instance, the European project Inteltex is developing a new, multifunctional textile that could be used as a wallpaper to detect temperature changes or chemical leakage or that could be used in medical and protective wear to monitor body temperature and mechanical stress. MIT's Institute for Soldier Nanotechnologies works on smart surfaces that switch properties. Nanotechnology-enabled smart materials are still very early days but basic progress is being made. Another small building block towards smart materials was recently reported by Italian researchers who demonstrated photo-switchable nanofibers based on the reversible transformation between two molecular photochemical states, exhibiting different chemico-physical characteristics.
Jan 31st, 2008
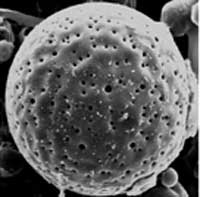 Hollow polymeric micro- and nanoparticles have numerous existing and many anticipated applications in drug delivery, ranging from the controlled release of drugs, cosmetics, inks, pigments or chemical reagents to the protection of biologically active species, and removal of pollutants. Encapsulation also allows drug targeting via cell and tissue-specific ligands. There is a variety of methods available for synthesis of polymer microspheres with hollow interiors. The hollow particles are most commonly prepared by coating the surfaces of colloidal templates with thin layers of the desired material (or its precursor), followed by selective removal of the templates by means of calcination or chemical etching. For polymers, methods such as emulsion polymerization, phase separation, cross-linking of micelles and self-assembly have also been demonstrated for generating hollow structures. The hollow polymer particles produced by these methods present either a closed-core-shell structure or many small pores on their surface. However, these synthetic approaches present limitations on the choice of polymers that can be produced as hollow microspheres. Also, the number, size and shape of the surface pores can not be easily manipulated. When these materials are used for encapsulation-related applications, the encapsulation of the desired functional materials is usually too slow and/or too labor-intense. These problems have motivated scientists to develop the synthesis of a new class of polymer microspheres, which they called microscale fish bowls. Their unique feature consists in the presence of one big pore on their surface that allows easy and fast diffusion of a functional material to be encapsulated. Another new feature is that this newly developed method presents no limitations on the choice of polymers that can be produced as microscale fish bowls.
Hollow polymeric micro- and nanoparticles have numerous existing and many anticipated applications in drug delivery, ranging from the controlled release of drugs, cosmetics, inks, pigments or chemical reagents to the protection of biologically active species, and removal of pollutants. Encapsulation also allows drug targeting via cell and tissue-specific ligands. There is a variety of methods available for synthesis of polymer microspheres with hollow interiors. The hollow particles are most commonly prepared by coating the surfaces of colloidal templates with thin layers of the desired material (or its precursor), followed by selective removal of the templates by means of calcination or chemical etching. For polymers, methods such as emulsion polymerization, phase separation, cross-linking of micelles and self-assembly have also been demonstrated for generating hollow structures. The hollow polymer particles produced by these methods present either a closed-core-shell structure or many small pores on their surface. However, these synthetic approaches present limitations on the choice of polymers that can be produced as hollow microspheres. Also, the number, size and shape of the surface pores can not be easily manipulated. When these materials are used for encapsulation-related applications, the encapsulation of the desired functional materials is usually too slow and/or too labor-intense. These problems have motivated scientists to develop the synthesis of a new class of polymer microspheres, which they called microscale fish bowls. Their unique feature consists in the presence of one big pore on their surface that allows easy and fast diffusion of a functional material to be encapsulated. Another new feature is that this newly developed method presents no limitations on the choice of polymers that can be produced as microscale fish bowls.
Jan 25th, 2008
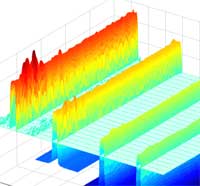 Sophisticated optical lithography techniques have been developed by the semiconductor industry to pack more and more transistors onto chips. On the road to a billion transistors per chip, Intel has already developed transistors so small that 200 million of them could fit on the head of a pin. As if that wasn't small enough, scientists are pushing further down, hoping to be able one day to reliably (and affordably) control surface features as small as 1 nm. With today's technology, cost-effective fabrication in the sub-50 nm range is a major challenge. Given the advanced development of (nano)lithography it is not surprising that various forms of it are the most common techniques used by nanotechnology researchers for manipulating sub-100 nm surface features. With the current state of optical lithography it appears that traditional commercial lithography techniques will not be cost effective below 30 nm. State-of-the-art electron beam lithography (EBL) has been proved to be capable of delivering resolution in the 10 nm range. Unfortunately, EBL is slow, very expensive and it is very unlikely that it can effectively go below 10 nm. The same limitations hold for x-rays and focused ion beams (FIBs), with additional tremendous difficulties in developing equipment for beam manipulation and focusing on nanometer scales.
Sophisticated optical lithography techniques have been developed by the semiconductor industry to pack more and more transistors onto chips. On the road to a billion transistors per chip, Intel has already developed transistors so small that 200 million of them could fit on the head of a pin. As if that wasn't small enough, scientists are pushing further down, hoping to be able one day to reliably (and affordably) control surface features as small as 1 nm. With today's technology, cost-effective fabrication in the sub-50 nm range is a major challenge. Given the advanced development of (nano)lithography it is not surprising that various forms of it are the most common techniques used by nanotechnology researchers for manipulating sub-100 nm surface features. With the current state of optical lithography it appears that traditional commercial lithography techniques will not be cost effective below 30 nm. State-of-the-art electron beam lithography (EBL) has been proved to be capable of delivering resolution in the 10 nm range. Unfortunately, EBL is slow, very expensive and it is very unlikely that it can effectively go below 10 nm. The same limitations hold for x-rays and focused ion beams (FIBs), with additional tremendous difficulties in developing equipment for beam manipulation and focusing on nanometer scales.
Jan 24th, 2008
 When two surfaces approach each other in air, they attract. A phenomenon that is explained by van der Waals forces that affect molecules' interaction. Without these - very weak - intermolecular forces, life as we know it would be impossible. They are responsible for a number of properties of molecular compounds, including crystal structures, condensing, melting and boiling points, surface tension, and densities. Intermolecular forces form molecules like enzymes, proteins, and DNA into the shapes required for biological activity. Van der Waals forces can also be repulsive, for instance when two surfaces approach each other in liquid: the same force which causes attraction in air (and which is responsible for so called stiction and adhesion) can be made repulsive by choosing the right combination of surface materials and intervening liquid. This force has the characteristic that it increases very rapidly with very small changes in separation when the surfaces are close to each other. Researchers now have shown that if repulsive van der Waals forces exist between two surfaces prior to their contact then friction is essentially precluded and supersliding is achieved. This opens the possibility that, in certain material systems, the controlled use of repulsive van der Waals forces could be a way to reduce, if not eliminate, friction.
When two surfaces approach each other in air, they attract. A phenomenon that is explained by van der Waals forces that affect molecules' interaction. Without these - very weak - intermolecular forces, life as we know it would be impossible. They are responsible for a number of properties of molecular compounds, including crystal structures, condensing, melting and boiling points, surface tension, and densities. Intermolecular forces form molecules like enzymes, proteins, and DNA into the shapes required for biological activity. Van der Waals forces can also be repulsive, for instance when two surfaces approach each other in liquid: the same force which causes attraction in air (and which is responsible for so called stiction and adhesion) can be made repulsive by choosing the right combination of surface materials and intervening liquid. This force has the characteristic that it increases very rapidly with very small changes in separation when the surfaces are close to each other. Researchers now have shown that if repulsive van der Waals forces exist between two surfaces prior to their contact then friction is essentially precluded and supersliding is achieved. This opens the possibility that, in certain material systems, the controlled use of repulsive van der Waals forces could be a way to reduce, if not eliminate, friction.
 Subscribe to our Nanotechnology Spotlight feed
Subscribe to our Nanotechnology Spotlight feed





