| Posted: Apr 02, 2014 | |
Smallest DNA origami nanorobot yet has a switchable flap |
|
| (Nanowerk Spotlight) One of the most important developments in structural DNA nanotechnology – where DNA molecules are assembled into organized and programmable structures – has been the use of a scaffold strand and hundreds of short staple strands for the assembly of three-dimensional (3D) DNA origami objects. These objects are similar in size to virus capsids and have gained a lot of interest as nanocontainers for drug delivery. Since they can be programed to carry out specific tasks (like transporting molecules and releasing them at a target site), these assemblies act like a robotic system – a nanorobot. | |
| Not to be confused with the nanorobots of science fiction, for medical nanotechnology researchers a nanorobot, or nanobot, is a popular term for molecules with a unique property that enables them to be programmed to carry out a specific task. | |
| In what is the smallest 3D DNA origami box so far, researchers in Italy have now fabricated a nanorobot with a switchable flap that, when instructed with a freely defined molecular message, can perform a specifically programmed duty. | |
| Slightly larger nanocontainers with a controllable lid have already been demonstrated by others to be suitable for the delivery of drugs or molecular signals, but this new cylindrical nanobot has an innovative opening mechanism. | |
| The team, led by Giuseppe Firrao, a professor in the Department of Agricultural and Environmental Sciences at the University of Udine, has published their findings in the March 20, 2014 online edition of Small ("A DNA Origami Nanorobot Controlled by Nucleic Acid Hybridization"). | |
| "While in previously developed objects the lids had a lock that can be removed, our container is equipped with a flap moved by an actuator that physically pulls it in the open position," Firrao tells Nanowerk. "It is like opening a window on a windy day: you can just unlock it and allow the wind to do the job or you can use your arm to pull the window, keep it firmly open, and eventually close it." | |
 |
|
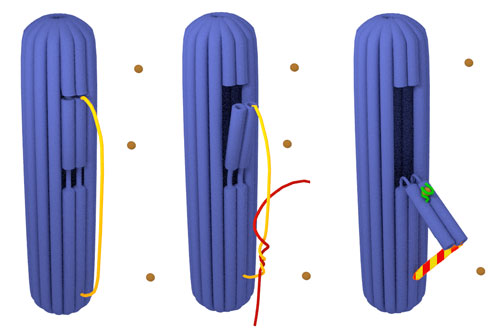
| Schematic model of the 3D DNA origami nanorobot with a switchable flap. Closed 3D DNA origami (blue) with the unreacted probe (yellow) (a). The 3D DNA origami during the hybridization probe (yellow) / target (red) (b) and of an actuated 3D DNA origami armed with hemin/G-quadruplex DNAzyme complex (c); the probe (yellow) hybridized to target (red), the flap opened (blue) and a DNAzyme (green) was pulled out of the cylinder and exposed to hemin (brown). (Image: Prof. Giuseppe Firrao, University of Udine) | |
| The reversibility of this actuation mechanism has been demonstrated for another nano object in a previous paper ("A Revertible, Autonomous, Self-Assembled DNA-Origami Nanoactuator"). Here, the team describes how they designed, constructed, and imaged a 100 nm-sized round DNA origami actuator capable of reversible motion in response to an external chemical stimulus. | |
| For their new work, the team used the flap mechanism developed in the previous work and reduced it in size to be included in a 3D origami of cylindrical shape. The resulting nanorobot had estimated dimensions of 14nm × 14nm × 48nm. The volume of the inside cavity (8nm × 8nm × 44nm) was estimated to be 3 zeptoliters. The flap dimensions were 9nm × 5nm. | |
| "As far as our nanorobot is concerned, the lumen is large enough to accommodate a single stranded nucleic acid, while the nano-object is small enough to be entirely contained within the capsid of viruses," says Firrao. "Our work therefore opens perspectives for the construction of nanorobots that can be hosted within cellular delivery vectors and can host, concurrently, selectively accessible molecular payloads in their internal cavity." | |
| The next steps in the team's research will be to monitor the flap opening with plasmonics and to operate the nanorobot in vivo. | |
| "By 'operate' we mean to control with external (e.g. light) or internal (e.g. development) events the reversible movement of the flap, in order to hide or display a DNAzyme, an enzyme, or a functional nucleic acid," explains Firrao. "This will be done in the perspective of manipulating cell responses to environmental stimuli, including disease." | |
| To gain precise control of opening and closing nanocontainers will be essential in the development of robust systems for the delivery of molecular signals into living organisms. This new work makes another step in this direction. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
