| Posted: Apr 14, 2014 | |
Researchers achieve complete control over on/off switching of the movement of a nanomachine |
|
| (Nanowerk Spotlight) Over the past few years, researchers have demonstrated that microtubules driven by kinesin – a motor protein that converts chemical energy, derived from the hydrolysis of adenosine triphosphate (ATP), into mechanical work – make flexible, responsive and effective molecular shuttles for nanotransport applications (read more: "Nanotechnology transport systems get a closer look"; for a broader overview on nanomotors and the design of nanotransportation systems read "Off to the races - artificial nanomachines are playing catch-up to nature's molecular motors"). | |
| In order to fully control these nanomotors it has to be possible to switch them on, switch them off, and regulate the speed and direction of their movements – achievements that researchers haven't fully attained yet. | |
| Several research labs have been working on building a fully controllable on/off switch mechanism of the motile properties of molecular machines such as kinesin-microtubules. Almost all of the methods achieved so far show non-reversible and incomplete switching, namely, just one-time switching from a 'fast to slow' or from 'slow to fast' state. Some research efforts accomplished reversible switching but in these cases the change in velocity was quite small. Most importantly, it has been impossible to stop the movement completely in such reversible switching methods. | |
| In new work from Professor Nobuyuki Tamaoki's laboratory at Hokkaido University it has now become possible, for the first time, to achieve complete control over on/off switching of the movement of a nanomachine. | |
| In a previous paper ("A non-nucleoside triphosphate for powering kinesin-microtubule motility with photo-tunable velocity"), Tamaoki's group already reported on their ongoing work on achieving reversible and repeated control over the motile properties of kinesin. | |
| In their new paper in the March 31, 2014 online edition of ACS Nano ("Complete ON/OFF Photoswitching of the Motility of a Nanobiomolecular Machine") the researchers investigated the photoresponsive inhibition properties of azobenzene-tethered peptides (azo-peptides) for the regulation of kinesin-driven microtubule motility. | |
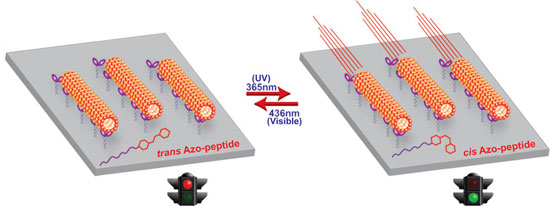 |
|
| Photoresponsive azo-peptide inhibitors completely stops and starts the motility of kinesin-microtubules with UV or visible photoirradiation. (Reprinted with permission from American Chemical Society) | |
| "We synthesized various compounds and finally discovered a new peptide-azobenzene – containing a peptide and a terminal azobenzene unit – that have a reverse order of the amino acids of the peptide from the kinesin’s tail," Tamaoki tells Nanowerk. " This compound completely stops and starts the motility of kinesin-microtubule. It also has a reasonably good on/off switchability with UV or visible photoirradiation for 1 second each." | |
| He notes that researchers have known that peptides whose amino acid sequence are taken from the tail part of kinesin show a moderate inhibition. "We thought about a covalent linkage between such peptide and photoisomerizable azobenzene, which we have been studying for a long time, to switch various molecular functions." | |
| The resulting photoresponsive azopeptide inhibitors can reversibly regulate microtubule motility over many cycles. If the concentration of the inhibitor is sufficiently high, the velocity of the microtubule can be stopped completely with photoirradiation. | |
| "Our photoresponsive molecular motor system enables us to make an active spot allowing cargo-attached microtubules to move by irradiating with UV light selectively at any desired region," says Tamaoki. "Such an active spot could be moved freely just by moving the position of UV light in an inactivated background irradiated with visible light. In such a manner, with a focused UV light, we would select one specific microtubule attached with cargos and guide it to a desired point. As a consequence, all kinds of transportation of nano-objects for separation, mixing, concentration would be possible." | |
| Research like this one will not only lead to new applications of motor proteins in artificial nano-transportation systems but also contribute to a better understanding of the exact mechanism of natural molecular machines (today, even the mechanism of the conversion of the chemical energy to work is not fully understood yet). | |
| "One of our goals is to change the energy source for the motility of bionanomachines from chemical energy to light energy in order to induce cycles of change in the molecular structure of kinesin," notes Tamaoki. "With the demonstration of our novel photoswitching compound we may have reached an important stage in our thinking about the mechanism of the motility of nanobiomachines." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
