| Posted: Jul 11, 2014 | |
DNA nanopyramids detect and combat bacterial infection |
|
| (Nanowerk Spotlight) Today's nanomedicine applications are dominated by platforms of synthetically manufactured materials – mostly nanoparticles of various elements and compositions – which require complex chemical manipulation. In addition, there is the lingering matter of potential toxicity to healthy cells during therapeutic application, which could occur due to the synthetic nature of these nanomaterials. | |
| One way to eliminate the toxicity issue is by using truly biocompatible natural carriers for sensing and drug delivery applications. The emerging field of DNA nanotechnology may provide a solution. In addition to acting as a scaffold to deliver therapeutic molecules, DNA nanostructures allow precise modification of the structure and presentation of these components (see for instance: "Vaccines developed from DNA nanostructures come one step closer to a clinical reality"). | |
| "In addition to the biocompatibility of DNA, the material also offers simplicity in its manipulation, high controllability in the end product size and shape, and modification versatility," David T. Leong, an assistant professor at the National University of Singapore (NUS)'s Department of Chemical and Biomolecular Engineering, explains to Nanowerk. "However, to date, there are very few studies that use DNA nanostructures as drug carriers and most of these studies are in anti-cancer applications." | |
| In this new work, motivated by the lack of an efficient theranostic platform for bacterial infection, the groups of David Leong and Jianping Xie co-developed a novel theranostic platform which is made by utilizing a self-assembled DNA nanopyramid (DP) as scaffold for incorporation of both detection and therapeutic moieties to combat bacterial infection. | |
| Reporting their findings in the June 18, 2014 online edition of ACS Applied Materials & Interfaces ("Novel Theranostic DNA Nanoscaffolds for the Simultaneous Detection and Killing of Escherichia coli and Staphylococcus aureus"), the researchers utilized DNA as their starting blocks to develop nanoparticles with theranostic capability, in which the nanoparticle possesses the capability to detect the presence of the bacteria infection and eradicate those infectious bacteria all in one package. | |
| "Commonly known as a genetic information repository, DNA is inherently a highly regulated polymer which could be tuned to have a precise size and shape by changing the DNA sequence," says Leong. "It is literally shape/size changing genetic engineering." | |
| In their study, the NUS team exploited this malleability of DNA to form self-assembled pyramidal DNA nanoparticles. | |
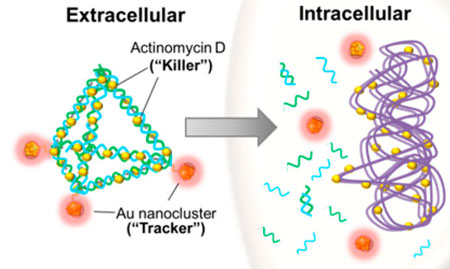
| They then loaded an active antimicrobial agent, Actinomycin D (AMD), by making use of the drug's innate properties to intercalate between DNA bases, as agents packed into the struts of the DNA pyramids. Based on the number of bases on each strut, the nanopyramids measure around 10 nm. | |
 |
|
| DNA based nanoparticle at work. Theranostic DNA nanopyramid was formed via a self-assembly process and was modified to carry Actinomycin D and gold nanoclusters, which act as the bacteria cells’ killer and tracker, respectively. Once taken in by the bacterial cell, this DNA based nanotheranostic particle were degraded further releasing its active antibacterial compound, Actinomycin D. (Reproduced with permission by American Chemical Society) | |
| As illustrated above, the scientists also coupled the DNA vertices with fluorescent gold nanoclusters which allowed them to detect the dead bacteria due to AMD that they delivered with this construct (DPAu/AMD). | |
| "Therefore, our DNA pyramid has served to be a simple, controllable, and biocompatible theranostic platform which can be used to combat bacterial infections," Leong concludes. | |
| He notes that this work offers new possibilities in the field of nanomedicine. As DNA is naturally available in every living creature, nanoparticles formed with DNA as the base material are truly biocompatible which could eliminate any possible toxicology that plagues several other nanomedicine platforms, especially ones based on inorganic materials or even those that contain heavy metals like cadmium. | |
| Moreover, by exploiting Watson-Crick base pairing, theoretically nanoparticles of any shape and size could be realized. Leong points out that this level of control is unique amongst polymeric material. | |
| "This control also allows us to design nanoparticles that are small enough (6-20 nm) to be taken up by bacterial cells, which are considerably smaller (ca. 1 µm) than mammalian cells, ensuring efficient drug delivery to the infectious bacteria in order to eradicate them." | |
| "Lastly" says Leong, "DNA bases offer many chemistry connections, allowing drug, protein, ligand, antibody, short oligonucleotide sequences or other small functional compounds to be specifically added onto the DNA nanoparticle; limited by our creativity." | |
| To assess the drug delivery ability of their DNA nanopyramids, loaded with Actinomycin D, they chose two bacterial strains, E. coli and S. aureus, which represent Gram-negative and Gram-positive groups, respectively. | |
| "Our results showed that both the nanopyramids alone as well as the nanopyramids coupled with the gold clusters did not exert any killing effect on the tested bacterial strains," says Leong. "Instead using the DPAu/AMD structure brought about a dose dependent killing effect on both E. coli and S. aureus cells. In addition, our result indicates that AMD packaged in DPAu show a significant killing effect of the bacteria when compared to the free AMD treatment." | |
| This method could be applied to deliver drugs to combat various infections and illnesses, as the DNA pyramid is versatile enough to carry various drugs, for example through intercalation (as here shown in the case of actinomycin D) and sequence specific-bound drugs. The geometric shapes also allow embedding of diagnostic materials which could be used to detect dead cells or bacteria, or to sense the biomarkers specific to certain diseases to be treated. | |
| Leong and his group see DNA as an important base material for the next generation of nanomedicine systems with a high level of achievable control of the major parameters that dictate nanoparticulate performance: "This DNA based nanomedicine platform will be efficient in term of drug loading and delivery as well as with lesser side effects. In addition, the ability to modify more modalities on this nanomedicine platform allows higher targeting specificities and detection." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
