| Posted: Jun 26, 2015 | |
New 'smart' nanoparticle platform for cancer thermotherapy |
|
| (Nanowerk Spotlight) Hyperthermia – a thermal therapy where body tissue is heated – has been used for decades to treat cancer. Because they are often poorly supplied with blood, cancerous tissues are more sensitive to increases in temperature compared to healthy tissues. Increasing the temperature of cancer cells beyond approximately 40°C is enough to induce programmed cell death, known as apoptosis. | |
| Microwave hyperthermia is one of the most important clinical thermotherapy techniques due to the instinctive advantages of non-intrusive heating model, fair depth of penetration in tissues and ideal potential of killing tumor cells without surgical risks or toxicity of chemotherapy. | |
| However, it is often difficult to apply heat locally to a tumor. Typically, limbs or other parts of the body that contain a tumor are heated externally. This often leads to considerable discomfort for the patient and damage to healthy tissues surrounding the tumor. | |
| By bringing together experts in medicine with experts in nanotechnology, researchers aim to explore the possibilities of designing microwave sensitive agents, which focus the destructive effects of microwaves specifically onto tumor cells. | |
| Scientists at the Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, have now proposed a novel approach to apply micro- and nanomaterials as microwave susceptible agents in tumor hyperthermia in vivo for the first time. | |
| "Our research shows that this approach inhibits tumor growth and markedly prolongs survival in animal models," Dr. Xianwei Meng, who led the research team, tells Nanowerk. | |
| The team reported their findings in Biomaterials ("Insights into a microwave susceptible agent for minimally invasive microwave tumor thermal therapy"). | |
| "Our microcapsules are composed of liquid core of sodium chloride (NaCl) solutions and biocompatible sodium alginate as wall materials," Meng explains. "In the presence of microwave, the absorbed microwave energy is transferred to the kinetic and interionic energies of ions and stored as Joule heating energy of salt ions via the interactions between salt ions, which leads to rapid temperature rise." | |
| Due to the excellent microwave susceptible properties and low bio-toxicity, the researchers achieved excellent therapy efficiency with a tumor inhibiting ratio of 97.85%. | |
| With a computer-simulated model, the team confirmed the mechanism of high microwave heating efficiency in theory, demonstrating that the spatial confinement efficiency of microcapsule walls endows the inside ions with high microwave susceptible properties. | |
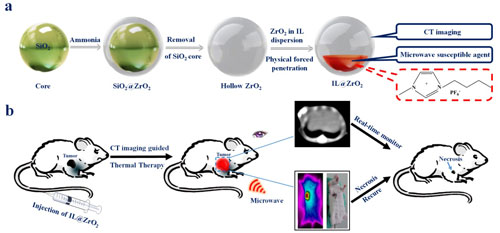
| While this all sounds great in theory, Professor Ke Xu from the Department of Radiology, First Hospital of China Medical University, joined the research effort. Subsequently, the team developed a simple hollow ZrO2 nanostructure as carrier to encapsulate ionic liquid (IL@ZrO2). | |
| This novel nanostructure integrates the X-ray computed tomography (CT) imaging properties of hollow ZrO2 nanoparticles and the high microwave heating efficiency of the ionic liquid core. | |
| "The ZrO2 shell produces an enhanced CT imaging effect which allows highly sensitive detection in vivo and in vitro, and the ionic liquid inside produces a microwave susceptibility effect which allows excellent thermotherapy efficiency," Meng summarizes the results. | |
 |
|
| (a) Schematic illustration of synthesizing hollow ZrO2 nanoparticles via template method and encapsulating IL via physical forced penetration method. (b) Schematic illustration of IL@ZrO2 as a multifunctional theranostic agent for CT imaging guided microwave thermal therapy in vivo and real-time monitoring the therapeutic outcomes visually. (Published by The Royal Society of Chemistry) (click on image to enlarge) | |
| The related findings were published in Chemical Science ("A smart all-in-one theranostic platform for CT imaging guided tumor microwave thermotherapy based on IL@ZrO2 nanoparticles"). | |
| This simple nanostructure can be used as a multifunctional theranostic agent via combining diagnostic and therapeutic modalities into one 'package'. | |
| In vitro and in vivo imaging results prove the potential of CT imaging application for real-time monitoring the biodistribution and metabolic process, and assessing the therapeutic outcomes, according to Meng. The therapeutic outcomes can be assessed visually using CT imaging. Meanwhile, real-time monitoring the biodistribution of ZrO2@IL in vivo can be achieved by enhanced CT imaging via mini swine models without batch experimental animals sacrificed different time points post injection reported in previous literatures. | |
| "We could completely inhibit tumor growth after just a single microwave thermotherapy with ultra low power (1.8 W, 450 MHz) in the animal model," Meng concludes. "We believe the novel thought will open a new era in the field of tumor hyperthermia field." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
