| Posted: Jun 28, 2016 | |
Overcoming adaptive resistance in cancer with a 2-in-1 nanoparticle approach |
|
| (Nanowerk Spotlight) Currently in the clinic and in clinical development, there is heavy emphasis placed on understanding how drugs can be 'complementary'. That is to say, what combination of drugs will be most effective in killing tumor cells and overcome potential resistance mechanisms. | |
| In fact, adding synergistic combinations of drugs is reshaping how cancer is treated, even in the context of immunotherapy. | |
| A new study just published in ACS Nano ("Rationally Designed 2-in-1 Nanoparticles Can Overcome Adaptive Resistance in Cancer") provides critical evidence that complementary drugs must be in spatial proximity to truly exert their synergistic potential. | |
| "This means, that in any context in which combination therapy is used, there must be a bioengineering approach – for example using designer nanoparticles or some method of tethered vehicle – to ensure that both drugs in the pair arrive within the same cell at the same time," Shiladitya Sengupta, an Assistant Professor of Health Sciences and Technology at Brigham and Women's Hospital, explains to Nanowerk. "This could very easily end the era of individual drug formulation." | |
 |
|
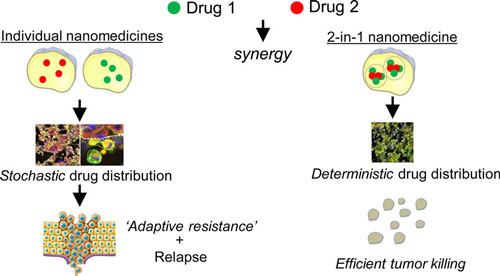
| Schematics of the proposed approach. (Reprinted with permission by American Chemical Society) | |
| Adaptive resistance is a mechanism that allows tumor cells to survive the initial onslaught of chemotherapy by mounting a phenotypic response – without any acquisition of a mutation – in response to therapy. This is a new concept in cancer therapy that researchers are increasingly realizing is a key driver of chemotherapy failure. | |
| One of the key findings in the team's study is that the use of 2-in-1 nanoparticles can help overcome adaptive resistance in cancer. They show how these resistant cells evolve,and that these 2-in-1 nanoparticles can prevent adaptive resistance. | |
| The other key finding is an answer to the question why two drugs delivered from one nanoparticle is better than a combination of two nanoparticles, each carrying a single drug. | |
| "This was an observation made in many previous studies," says Sengupta. "We show that when a combination of two nanoparticles is used, there is a stochastic distribution of the nanoparticles to the cells, i.e. while a majority of cells receives both the nanoparticles (i.e. drugs), there are some cells that get only one of the nanoparticles. This means the combination effect will not be achieved in these cells. This is overcome by a 2-in-1 nanoparticle, where every cell receives the combination therapy." | |
| From a biological perspective, the scientists were initially interested whether a cancer cell will stochastically acquire resistance – i.e. drug pressure randomly transforms sensitive cancer cells to a resistant phenotype – or if the acquisition of a drug tolerant/resistant phenotype is acquired through a defined trajectory, i.e. deterministic intracellular program. | |
| Such an understanding has never been tested before. The team used a combination of empirical data and computational biology to build a real-time view of the resistance development program, and confirmed by mathematical certainty that cancer cells are able to overcome therapy pressure by transforming their cell state by a defined program. | |
| "It was previously thought that only small subpopulations of cells will overcome therapy pressure and survive," Sengupta points out. "Our evidence suggests that cells will acquire resistance to therapy as a consequence of the drug itself, for example PI3K driving acquired tolerance/resistance to a taxane." | |
| He explains that in order to destroy the resistance mechanism, inhibitors of PI3K must be present within this cell before the process of resistance can be completed. | |
| "In the context of bioengineering, we asked how this knowledge can be exploited to design next-generation nanoparticles," Sengupta notes. "It is not easy to achieve drug loading of 2 drugs in a nanoparticle, We used computational design to engineer these nanoparticles." | |
| As a proof of concept study, the team describes two test cases in which a pair of drugs – spatially and temporally controlled – exert synergistic potential in the context of drug resistance, primarily in triple negative breast cancer. | |
| It should be noted that cancer is a heterogeneous disease, and the phenotypic and genomic landscape within and between tumors is extraordinarily complex. | |
| Resistance mechanisms are not singular, they are generally as diverse as the tumor itself. Researchers still need to unravel the evolutionary mechanisms that enable cancer cells to resist treatment in the context of heterogeneity and the stromal microenvironment. | |
| The team's subsequent studies will be focused on developing appropriate pairs of drugs, designed within single nanoscale technologies, to personalize medicine in the case of unique tumor landscapes. | |
| For example, gamma-secretase inhibitors have been shown to upregulate mTOR, which highlights the combined use of g-secretase inhibitors and mTOR inhibitors as complementary drug pairs in pediatric leukemia. | |
| The scientists are addressing how such complementary pairs can be harnessed to overcome resistance mechanisms in different tumor types. | |
| "We believe that our findings solve an unrecognized problem in the treatment of cancer," Sengupta concludes. "That is, complementary drug pairs must be formulated into single vehicles so that resistance cannot develop. There are currently more than 250 clinical trials that study combinations of drugs. Many of these trials could benefit with greater efficacy if the drug pairs were tied together using such an approach that we have presented in our study; i.e. 2-in-1 supramolecular nanomedicines. Such an advance in our treatment of cancer could profoundly affect the rates of relapse and mortality." | |
| There is a clear role for immune cells, tumor associated macrophages, fibroblasts and endothelial cells in the mechanisms that allow cancer cells to evade therapy pressure. Scientists have only begun their travel on a long road to build a bridge between their understanding of the biology of cancer and drug resistance on one hand and emerging bioengineering approaches on the other. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
