| Posted: Apr 16, 2009 | |
Size matters. Comparing the toxicity of micro- to nanoparticles |
|
| (Nanowerk Spotlight) There is a slowly growing body of work that investigates the toxicity of synthesized nanoparticles to plants, aquatic invertebrates, algae, bacteria and different cell lines and we have been covering this topic in previous Spotlights as well as our nanoRISK newsletter. Although the potential negative effects of nanoparticles on organisms and the environment have raised concerns, limited studies so far have examined the difference between nanoparticles and bulk particles with the same chemical composition and mineral phase, or addressed the toxicity of dissolved metal ions from the nanoparticles. | |
| In new work by scientists at the University of Massachusetts, the toxicity of bulk oxide particles and the released ions were assessed along with four oxide nanoparticles, which clearly showed that size matters. | |
| Baoshan Xing, a professor in the Department of Plant, Soil & Insect Sciences at the University of Massachusetts, and his group examined nanoparticulate aluminum oxide (60 nm), silicon dioxide (20 nm), titanium dioxide (50 nm), and zinc oxide (20 nm) – nanoparticles that are common industrial additives and have various applications ranging from cosmetics to electronics. | |
| "We performed our experiments to better understand whether the bacterial toxicity of nanoparticles was size- or composition-related," Xing tells Nanowerk. "We did this by comparing the nanoparticles to their bulk counterparts, and by evaluating the contribution of dissolved metal ions to the overall toxicity. In addition, we examined the interaction between nanoparticles and bacteria and their surface properties." | |
| Xing's team conducted their toxicity assessments using three model bacteria species: gram-positive Bacillus subtilis; gram-negative Escherichia coli and Pseudomonus fluorescens. | |
| Since bacteria perform many critical roles in ecosystem function and productivity, the potential impact on them by nanoparticles released into the environment deserves particular attention. | |
| "Interactions between bacteria and nanoparticles may provide us with more information about the impact of nanoparticles on the ecosystem once released," says Xing. "At the same time, bacteria as single cell organisms are good test models to study the toxicity of nanoparticles and to explore how nanoparticles affect the cell/organism function." | |
| In this work, which has been published online in Environmental Pollution ("Bacterial toxicity comparison between nano- and micro-scaled oxide particles"), the team (working with Xing were Wei Jiang as first author and Hamid Mashayekhi) reports three major findings: | |
| 1) Aluminum oxide, silicon dioxide, and zinc oxide nanoparticles were toxic to the three bacteria species tested, while titanium dioxide nanoparticles did not show any observable toxicity. The zinc oxide nanoparticles showed the highest toxicity. | |
| 2) Aluminum oxide, silicon dioxide, and zinc oxide nanoparticles showed higher toxicity compared to their bulk particle counterparts. | |
| 3) Toxicity of nanoparticles was not only from the dissolved metal ions, but also from their greater tendency to attach to the bacterial cell walls. | |
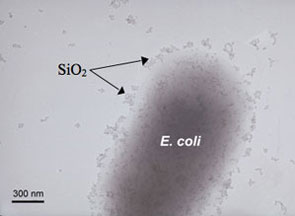  |
|
| Left: Adhesion of silicon dioxide nanoparticles on the E. coli as observed by TEM. Right: Adhesion of silicon dioxide nanoparticles on the E. coli as observed by AFM. (Images: Dr. Baoshan Xing, University of Massachusetts) | |
| "What we found shows that nanoparticles that were toxic to bacteria behave differently in bacterial suspension than the non-toxic ones" explains Xing. "The toxic nanoparticles could cover the whole bacteria surface, while the non-toxic ones under our experimental condition could not cover the bacteria cells but instead lumped together. The bacteria could attract the positively charged individual particles or small aggregates from the solution. The dispersion may increase both the mobility and toxicity of nanoparticles. Therefore the nanoparticles staying in aggregates were not toxic, while the dispersed nanoparticles could be attracted by bacteria and subsequently showed toxicity." | |
| The reason why only titanium dioxide didn't show toxicity among the four tested nanoparticle species is that it was the only highly negatively charged nanoparticle – the negatively charged bacterial cells repel the titanium dioxide nanoparticles. | |
| Xing notes that bacteria will attract positively charged nanoparticles from aquatic systems, increasing the chance of such nanoparticles entering the aquatic food chain. | |
| Since the the three bacteria species used in the experiments are widely present in soil and aquatic systems, they may be developed as indicators for nanoparticle toxicity. | |
| "The relationship between nanoparticle toxicity and attachment to bacteria found in our research may inspire the manufacturers of nanoparticles to develop environment-friendly nanoparticles by adjusting their surface properties," says Xing. "On the other hand, the nanoparticles' antimicrobial activity may be applied in developing self-sterilizing materials." | |
| So far the least understood aspects of nanoparticle toxicity with bacteria have to do with the mechanism of this toxicity. Most researchers believe that the toxicity to bacteria is caused by damage to the cell wall. | |
| The main challenge to mechanistic studies like the one conducted by the University of Massachusetts scientists is the lack of effective and sensitive enough methods to probe the changes to the bacterial cell wall (which is a very complicated and integrated structure). | |
| "It is very difficult to determine in which cell component the changes occur when we study the nanoparticles' effects using living cells," says Xing. "For most techniques, it is difficult to differentiate if the instrument signals are from the cell wall or the cell interior. And if instead we work on components extracted from bacterial cells, the observed effect may not represent what is occurring in integrated living cells." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
