| Posted: Nov 28, 2011 | |
Catch and release sensing of single malaria-infected red blood cells |
|
| (Nanowerk Spotlight) Graphene research papers are popping up left and right at what seems like an accelerating speed and growing volume. One of the areas that is seeing vast research interest is the biological interfacing of graphene for instance for sensor applications (see for example our recent Nanowerk Spotlight on graphene transistors to record action potential from cells). | |
| Today, we are looking at another exciting graphene bio application where a graphene sensor is integrated with microfluidics to sense malaria-infected red blood cells at the single-cell level. | |
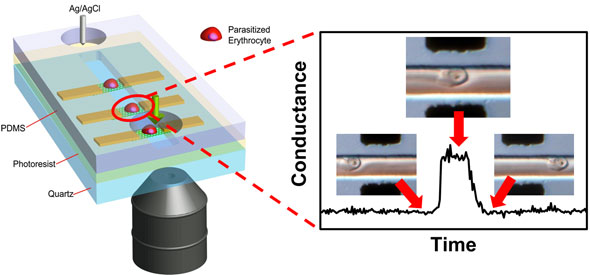
| "Graphene's attributes – good interface with living cells and biorecognition proteins; atomic thinness; optical transparency; current stability; and electrical sensitivity – enabled us to design and fabricate a selective flow-catch-release sensing platform, in which, for the first time, malaria-infected red blood cells can be optically differentiated without the need for fluorescence staining and simultaneously, electrically detected by graphene one cell at a time in a microfluidic channel," Professor Kian Ping Loh, a key member of the Graphene Research Centre at National University of Singapore (NUS), tells Nanowerk. The work was carried out by doctorate student Priscilla Ang, in collaboration with Professor Chwee Teck Lim's group at NUS' Division of Bioengineering. | |
| Loh and his collaborators report in the November 11, 2011 online edition of Nano Letters ("Flow Sensing of Single Cell by Graphene Transistor in a Microfluidic Channel"), this graphene sensor is able to generate dynamic disease diagnostic patterns in terms of conductance changes and characteristic dwell times. | |
 |
|
| Schematic illustration of an array of graphene transistors in a microfluidic channel through which cells flow. Specific binding between ligands on positively charged knobs of infected red blood cells and receptors functionalized on graphene induces a distinct conductance change. Conductance returns to baseline value when infected cell exits the graphene channel. (Image: Priscilla Ang, NUS) | |
| The main motivation for designing such a device lies in the fact that pathological cell-cell interaction is essentially mediated by a charge-based phenomenon. Any disease-induced structural changes will result in a noticeable change in the overall cell surface charge density, which can be used as an additional parameter for differentiation of disease state. | |
| "Hitherto, most disease detection and diagnostic tools rely on microscopy and antibody staining which require specialized training and may be succumb to misdiagnosis due to human errors," explains Loh. "While polymerase chain reaction (PCR) remains the gold standard, it is unsuitable for routine use due to its high operating cost. Besides these challenges, the heterogeneity of cellular behaviors in a large population of cells also makes such measurements insensitive to changes occurring in individual cells." | |
| Therefore, as Loh points out, detecting a change in cell surface charge at the single cell level presents a new alternative and complementary method for disease detection and diagnosis. As the NUS team has successfully demonstrated, this can be done by employing an array of graphene transistors, in which any change to the cell surface charge translates to a distinct change in the transconductance of the graphene. Moreover, easy integration with microfluidics enables the manipulation, isolation and statistical analysis of individual diseased cells. | |
| Malaria is among the most deadly infectious diseases on the planet. The hallmarks of an infection by P. falciparum – one of the most virulent parasite strains – include several irreversible structural modifications of the parasitized red blood cells. During an infection, these structural modifications cause the cytoadherence of affected red blood cells to endothelial cells lining the blood vessels and capillaries. CD36 receptor proteins on the endothelial cells are involved in this specific interaction. | |
| The researchers exploited this for their flow-catch-release scheme by functionalizing graphene with endothelial CD36 receptors for the selective capture of the malaria-infected cells when the diseased blood – consisting of a mixture of healthy and infected red blood cells – flows through a microfluidic channel. | |
| In addition to electrical sensing (a positively gated graphene will exhibit an increase in its channel conductance upon the binding of the infected cell because of electrostatic doping), the optical transparency of graphene allows the simultaneous optical monitoring of binding events via differential interference contrast (DIC) microscopy. | |
| "Analyzing the cell surface charge density will shed new insights into cell-cell interaction governing pathophysiology in near physiological conditions," Loh outlines the possibilities of this sensing approach. "In addition, our device is robust and can be employed to study and detect any kind of diseased cell. The ability to do a statistical percentage count of the infected cells as the population of infected and healthy cells flow through the graphene transistors in a microfluidic channel exhibits great promise for clinical diagnostic applications." | |
| There are many challenges ahead – for instance the economical production of high quality graphene – before this device can be commercialized. However, Loh and his team can envision the incorporation of graphene transistors in a lab-on-a-chip device, which allows high-throughput flow sensing of infected cells with small volume consumption and automated electrical readout for disease detection and diagnosis. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
