| Posted: Mar 03, 2008 | |
New nanocarriers for cancer treatment |
|
| (Nanowerk Spotlight) Since their discovery in 1961, liposomes - nanoscale vesicles composed of phospholipids - have been developed as nano-vectors that are used for a variety of biomedical applications including diagnostic imaging, gene therapy, biosensing and targeted drug delivery. In fact, the FDA-approved drugs Ambisome, Doxil and DaunoXome all contain liposomal formulations. Such liposomes are typically comprised of glycerol-based phospholipids that contain a hydrophilic (water-soluble) head-group and one or two hydrophobic (water-insoluble) hydrocarbon chains of varying length. In aqueous solution, these phospholipids self-assemble into a lipid bilayer, with the hydrophilic lipid groups oriented toward the aqueous solution and the hydrophobic groups protected in the bilayer's interior. The bilayers form spherical vesicles that are used to carry drugs and diagnostic imaging agents to sites of interest within the body. The hollow interior of the vesicles is hydrophilic and can easily encapsulate a variety of hydrophilic drugs or imaging agent molecules, which are then released from the liposomes in a controlled fashion. | |
| But what about hydrophobic molecules - those that aren't water-soluble and therefore aren't easily encapsulated within the interior of traditional phospholipid liposomes? Many beneficial, yet water-insoluble drugs do exist, but the current methods used to administer to these drugs to patients, such as dissolving them in alcohols, castor oil or other hydrophobic liquids for injection, can cause patients much discomfort or other side effects. For these reasons, the development of a nano-vector with a hydrophobic interior - one that could successfully encapsulate and release hydrophobic molecules - is of great interest to the nanomedicine community. | |
| Developing such a hydrophobic nano-vector is a major research focus for Dr. Ranga Partha, a biophysicist at the University of Texas Health Science Center in Houston, TX (UTHSC-H). Along with Dr. Jodie Conyers of UTHSC-H and Dr. Andreas Hirsch of Friedrich-Alexander University in Erlangen, Germany, Dr. Partha has recently reported the assembly of a nano-vector that might be capable of retaining hydrophobic molecules ("Self assembly of amphiphilic C60 fullerene derivatives into nanoscale supramolecular structures" – open access article). | |
| This nano-vector is easily formed from the self-assembly of chemically modified fullerenes into spherical vesicles of 50–100 nm diameter. Dr. Partha and his colleagues have termed these vesicles "buckysomes," a combination of the words "buckyballs" (a nickname for fullerenes) and "liposomes." | |
  |
|
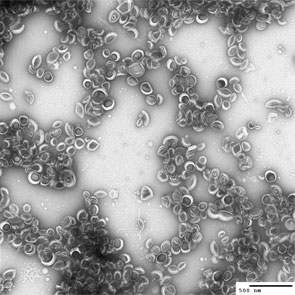
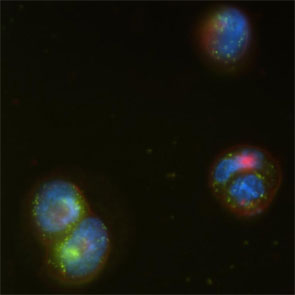
| Left: Negative-stain transmission electron microscopy image of buckysomes. Scale bar is 500 nm. The dark regions in the middle of some buckysomes are due to the uranyl acetate staining agent. Right: Fluorescence microscopy image of human epidermoid carcinoma cells incubated with fluorescein-buckysomes (green) for 18 hours, showing cell internalization with no change in localization following several washes with buffer. The cells themselves are counterstained (nuclei, blue; membranes, red). The image shown here is that of the blue, green and red emissions superimposed. (Images: Dr. Partha, University of Texas Health Science Center at Houston) | |
| The fullerene compound, referred to as AF-1, consists of a fullerene covalently attached to a dendritic group of 18 carboxylic acids and five pairs of 12-carbon esters (dodecyl malonates). Fullerenes are naturally hydrophobic, but the covalent attachment of these functional groups makes AF-1 amphiphilic (both water-soluble and insoluble), permitting its self-assembly into buckysomes containing both hydrophobic and hydrophilic regions, like a conventional liposome. However, unlike conventional liposomes, preliminary data have shown that these buckysomes are able to uptake hydrophobic molecules, making the buckysomes a promising candidate as a nano-vector for hydrophobic drug delivery. | |
| Dr. Partha and Dr. Conyers are currently studying the uptake and release of hydrophobic molecules by the buckysomes using hydrophobic dye molecules. Specifically, the release of the dye as function of varying pH in the buckysome solution is being investigated in hopes that pH control may be used to trigger the release of hydrophobic drugs from the buckysomes at specified sites within the body. | |
| The pH-controlled release of drugs from the buckysomes would be particularly useful for cancer treatment, since normal physiological pH values are typically near 7.0, but the pH inside malignant tumors is often lower (∼5.5 - 6.0). Dr. Partha expects that the buckysomes will become structurally destabilized at these lower pH values, thereby releasing their hydrophobic payloads. He plans to encapsulate hydrophobic anticancer drugs within the buckysomes to study their pH-triggered, targeted release at tumor sites. | |
| Additionally, the buckysomes appear to be nontoxic toward cells as determined by in vitro studies of cellular proliferation and membrane integrity of human kidney, liver and macrophage cells. These cells showed little difference upon exposure to the buckysomes compared to control exposures to buffer solutions. | |
| The buckysomes have also been proven to be localized within human coronary artery endothelial cells without inducing significant changes to the cells' morphology. Furthermore, preliminary data have demonstrated that the buckysomes are effectively internalized by carcinoma cells. Therefore, Dr. Partha expects that the buckysomes should be effective in their delivery of hydrophobic drugs to cancerous cells, and he plans to test this hypothesis using breast cancer tumor cells. | |
| Ongoing studies in Dr. Conyers' laboratories are also aimed at obtaining a deeper understanding of the buckysomes' cellular internalization mechanisms. | |
| By Dr. Jennifer Lyon, University of Texas Health Science Center at Houston | |
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
